Benzodiazepines, colloquially called "benzos", are a class of depressant drugs whose core chemical structure is the fusion of a benzene ring and a diazepine ring. They are prescribed to treat conditions such as anxiety disorders, insomnia, and seizures. The first benzodiazepine, chlordiazepoxide (Librium), was discovered accidentally by Leo Sternbach in 1955 and was made available in 1960 by Hoffmann–La Roche, who soon followed with diazepam (Valium) in 1963. By 1977, benzodiazepines were the most prescribed medications globally; the introduction of selective serotonin reuptake inhibitors (SSRIs), among other factors, decreased rates of prescription, but they remain frequently used worldwide.

Diazepam, first marketed as Valium, is a medicine of the benzodiazepine family that acts as an anxiolytic. It is commonly used to treat a range of conditions, including anxiety, seizures, alcohol withdrawal syndrome, muscle spasms, insomnia, and restless legs syndrome. It may also be used to cause memory loss during certain medical procedures. It can be taken by mouth, inserted into the rectum, injected into muscle, injected into a vein or used as a nasal spray. When given into a vein, effects begin in one to five minutes and last up to an hour. By mouth, effects begin after 15 to 60 minutes.

Temazepam is a medication of the benzodiazepine class which is generally used to treat severe or debilitating insomnia. It is taken by mouth. Temazepam is rapidly absorbed, and significant hypnotic effects begin in less than 30 minutes and can last for up to eight hours. Many studies, some going as far back as the early 1980s out of Australia and the United Kingdom, both of which have had serious temazepam abuse epidemics and related mortality, have all mostly corroborated each other and proven that the potential for abuse and physical dependence is very high, even in comparison to many other benzodiazepines. As a result, prescriptions for hypnotics such as temazepam have seen a dramatic decrease since 2010, while anxiolytics such as alprazolam (Xanax), clonazepam, and lorazepam (Ativan) have increased or remained stable. Temazepam and similar hypnotics, such as triazolam (Halcion) are generally reserved for severe and debilitating insomnia. They have largely been replaced by z-drugs and atypical antidepressants as first line treatment for insomnia.

Triazolam, sold under the brand name Halcion among others, is a central nervous system (CNS) depressant tranquilizer of the triazolobenzodiazepine (TBZD) class, which are benzodiazepine (BZD) derivatives. It possesses pharmacological properties similar to those of other benzodiazepines, but it is generally only used as a sedative to treat severe insomnia. In addition to the hypnotic properties, triazolam's amnesic, anxiolytic, sedative, anticonvulsant, and muscle relaxant properties are pronounced as well.

Clonazepam, sold under the brand names Klonopin and Rivotril, is a medication used to prevent and treat anxiety disorders, seizures, bipolar mania, agitation associated with psychosis, and akathisia. It is a tranquilizer of the benzodiazepine class. It possesses anxiolytic, anticonvulsant, sedative, hypnotic, and skeletal muscle relaxant properties. It is typically taken by mouth. Effects begin within one hour and last between six and twelve hours.

Oxazepam is a short-to-intermediate-acting benzodiazepine. Oxazepam is used for the treatment of anxiety and insomnia and in the control of symptoms of alcohol withdrawal syndrome.

Nonbenzodiazepines, sometimes referred to colloquially as Z-drugs, are a class of psychoactive drugs that are benzodiazepine-like in uses, such as for treating insomnia and anxiety.
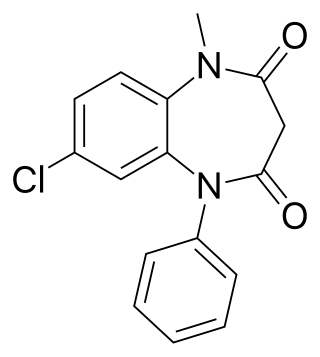
Clobazam, sold under the brand names Frisium, Onfi and others, is a benzodiazepine class medication that was patented in 1968. Clobazam was first synthesized in 1966 and first published in 1969. Clobazam was originally marketed as an anxioselective anxiolytic since 1970, and an anticonvulsant since 1984. The primary drug-development goal was to provide greater anxiolytic, anti-obsessive efficacy with fewer benzodiazepine-related side effects.

Loprazolam (triazulenone) marketed under many brand names is a benzodiazepine medication. It possesses anxiolytic, anticonvulsant, hypnotic, sedative and skeletal muscle relaxant properties. It is licensed and marketed for the short-term treatment of moderately-severe insomnia.

Adinazolam is a tranquilizer of the triazolobenzodiazepine (TBZD) class, which are benzodiazepines (BZDs) fused with a triazole ring. It possesses anxiolytic, anticonvulsant, sedative, and antidepressant properties. Adinazolam was developed by Jackson B. Hester, who was seeking to enhance the antidepressant properties of alprazolam, which he also developed. Adinazolam was never FDA approved and never made available to the public market; however, it has been sold as a designer drug.

Etizolam is a thienodiazepine derivative which is a benzodiazepine analog. The etizolam molecule differs from a benzodiazepine in that the benzene ring has been replaced by a thiophene ring and triazole ring has been fused, making the drug a thienotriazolodiazepine.

Chlordiazepoxide, trade name Librium among others, is a sedative and hypnotic medication of the benzodiazepine class; it is used to treat anxiety, insomnia and symptoms of withdrawal from alcohol and other drugs.

Delorazepam, also known as chlordesmethyldiazepam and nordiclazepam, is a drug which is a benzodiazepine and a derivative of desmethyldiazepam. It is marketed in Italy, where it is available under the trade name EN and Dadumir. Delorazepam (chlordesmethyldiazepam) is also an active metabolite of the benzodiazepine drugs diclazepam and cloxazolam. Adverse effects may include hangover type effects, drowsiness, behavioural impairments and short-term memory impairments. Similar to other benzodiazepines delorazepam has anxiolytic, skeletal muscle relaxant, hypnotic and anticonvulsant properties.

Alcohol detoxification is the abrupt cessation of alcohol intake in individuals that have alcohol use disorder. This process is often coupled with substitution of drugs that have effects similar to the effects of alcohol in order to prevent alcohol withdrawal. When withdrawal does occur, it results in symptoms of varying severity.

Abecarnil (ZK-112,119) is an anxiolytic drug from the β-Carboline family. It is one of a relatively recently developed class of medicines known as the nonbenzodiazepines, which have similar effects to the older benzodiazepine group, but with quite different chemical structures. It is a partial agonist acting selectively at the benzodiazepine site of the GABAA receptor.
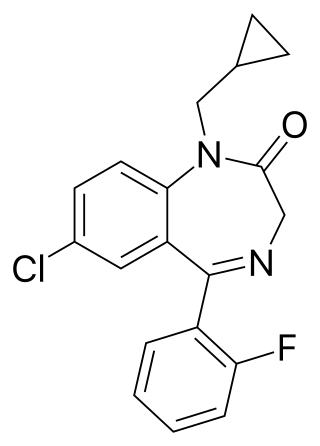
Flutoprazepam (Restas) is a drug which is a benzodiazepine. It was patented in Japan by Sumitomo in 1972 and its medical use remains mostly confined to that country. Its muscle relaxant properties are approximately equivalent to those of diazepam - however, it has more powerful sedative, hypnotic, anxiolytic and anticonvulsant effects and is around four times more potent by weight compared to diazepam. It is longer acting than diazepam due to its long-acting active metabolites, which contribute significantly to its effects. Its principal active metabolite is n-desalkylflurazepam, also known as norflurazepam, which is also a principal metabolite of flurazepam.

Metaclazepam is a drug which is a benzodiazepine derivative. It is a relatively selective anxiolytic with less sedative or muscle relaxant properties than other benzodiazepines such as diazepam or bromazepam. It has an active metabolite N-desmethylmetaclazepam, which is the main metabolite of metaclazepam. There is no significant difference in metabolism between younger and older individuals.

Pipequaline (INN) is an anxiolytic drug that was never marketed. It possesses a novel chemical structure that is not closely related to other drugs of this type. The drug has a similar pharmacological profile to the benzodiazepine family of drugs, but with mainly anxiolytic properties and very little sedative, amnestic or anticonvulsant effects, and so is classified as a nonbenzodiazepine anxiolytic.

Premazepam is a benzodiazepine derivative. It is a partial agonist of benzodiazepine receptors and was shown in 1984 to possess both anxiolytic and sedative properties in humans but was never marketed.

Benzodiazepine dependence defines a situation in which one has developed one or more of either tolerance, withdrawal symptoms, drug seeking behaviors, such as continued use despite harmful effects, and maladaptive pattern of substance use, according to the DSM-IV. In the case of benzodiazepine dependence, however, the continued use seems to be associated with the avoidance of unpleasant withdrawal reaction rather than from the pleasurable effects of the drug. Benzodiazepine dependence develops with long-term use, even at low therapeutic doses, without the described dependence behavior.