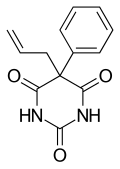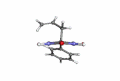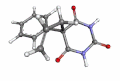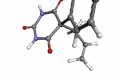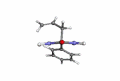
Picrotoxin, also known as cocculin, is a poisonous crystalline plant compound. It was first isolated by the French pharmacist and chemist Pierre François Guillaume Boullay (1777–1869) in 1812. The name "picrotoxin" is a combination of the Greek words "picros" (bitter) and "toxicon" (poison). A mixture of two different compounds, picrotoxin occurs naturally in the fruit of the Anamirta cocculus plant, although it can also be synthesized chemically.

Phenobarbital, also known as phenobarbitone or phenobarb, sold under the brand name Luminal among others, is a medication of the barbiturate type. It is recommended by the World Health Organization (WHO) for the treatment of certain types of epilepsy in developing countries. In the developed world, it is commonly used to treat seizures in young children, while other medications are generally used in older children and adults. In developed countries it is used for veterinary purposes. It may be used intravenously, injected into a muscle, or taken by mouth. The injectable form may be used to treat status epilepticus. Phenobarbital is occasionally used to treat trouble sleeping, anxiety, and drug withdrawal and to help with surgery. It usually begins working within five minutes when used intravenously and half an hour when administered by mouth. Its effects last for between four hours and two days.

Citronellol, or dihydrogeraniol, is a natural acyclic monoterpenoid. Both enantiomers occur in nature. (+)-Citronellol, which is found in citronella oils, including Cymbopogon nardus (50%), is the more common isomer. (−)-Citronellol is widespread, but particularly abundant in the oils of rose (18–55%) and Pelargonium geraniums.
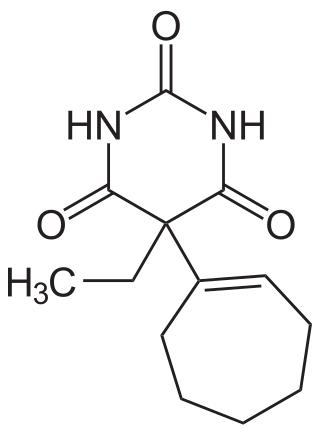
Heptabarb, also known as heptabarbitone (BAN) or heptabarbital, is a sedative and hypnotic drug of the barbiturate family. It was used in Europe for the treatment of insomnia from the 1950s onwards, but has since been discontinued.

Monobactams are bacterially-produced monocyclic β-lactam antibiotics. The β-lactam ring is not fused to another ring, in contrast to most other β-lactams.

Hexobarbital or hexobarbitone, sold both in acid and sodium salt forms as Citopan, Evipan, and Tobinal, is a barbiturate derivative having hypnotic and sedative effects. It was used in the 1940s and 1950s as an agent for inducing anesthesia for surgery, as well as a rapid-acting, short-lasting hypnotic for general use, and has a relatively fast onset of effects and short duration of action. It was also used to murder women prisoners at Ravensbrück concentration camp. Modern barbiturates have largely supplanted the use of hexobarbital as an anesthetic, as they allow for better control of the depth of anesthesia. Hexobarbital is still used in some scientific research.

Molten salt is salt which is solid at standard temperature and pressure but liquified due to elevated temperature. A salt that is liquid even at standard temperature and pressure is usually called a room-temperature ionic liquid, and molten salts are technically a class of ionic liquids.

Aprobarbital, sold as Oramon, Somnifaine, and Allonal, is a barbiturate derivative invented in the 1920s by Ernst Preiswerk. It has sedative, hypnotic and anticonvulsant properties, and was used primarily for the treatment of insomnia. Aprobarbital was never as widely used as more common barbiturate derivatives such as phenobarbital and is now rarely prescribed as it has been replaced by newer drugs with a better safety margin.

Cyclopentobarbital sodium is a barbiturate derivative invented in the 1940s. It has sedative and anticonvulsant properties, and was used primarily as an anaesthetic in veterinary medicine. Cyclopal is considered similar in effects to phenobarbital but lasts almost three times as long, and is considered a long-acting barbiturate with a fairly slow onset of action.

Barbiturates are a class of depressant drugs that are chemically derived from barbituric acid. They are effective when used medically as anxiolytics, hypnotics, and anticonvulsants, but have physical and psychological addiction potential as well as overdose potential among other possible adverse effects. They have been used recreationally for their anti-anxiety and sedative effects, and are thus controlled in most countries due to the risks associated with such use.

Fenpentadiol (INN), also known as phenpentanediol, is a drug described as a tranquilizer and antidepressant that was formerly marketed in Europe. It also has stimulant, sedative, and anxiolytic effects, with the latter two occurring only at higher doses.

Barbiturate overdose is poisoning due to excessive doses of barbiturates. Symptoms typically include difficulty thinking, poor coordination, decreased level of consciousness, and a decreased effort to breathe. Complications of overdose can include noncardiogenic pulmonary edema. If death occurs this is typically due to a lack of breathing.
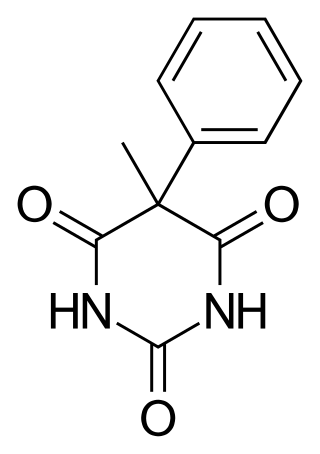
Heptobarbital (Rutonal), also known as phenylmethylbarbituric acid is a barbiturate derivative. It has often been confused with methylphenobarbital because both drugs contain a methylphenyl moiety and are overall very similar in structure.
The Zwikker reagent is used as a simple spot-test to presumptively identify barbiturates. It is composed of a mixture of two solutions. Part A is 0.5 g of copper (II) sulfate in 100 ml of distilled water. Part B consists of 5% pyridine (v/v) in chloroform. One drop of each is added to the substance to be tested and any change in colour is observed.

Thiotetrabarbital is a drug which is a short-acting barbiturate derivative that is used as an anesthetic. It has been used in veterinary medicine.
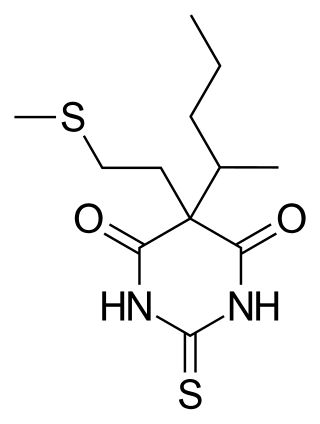
Methitural, or methitural sodium, also known as methioturiate, is a barbiturate derivative which was marketed in the 1950s in Europe as an ultra-short-acting intravenous anesthetic.

Propylbarbital, also known as 5,5-dipropylbarbituric acid, is a barbiturate derivative used as a hypnotic drug.

Buthalital sodium, or buthalitone sodium (BAN), is a barbiturate derivative which was under development as a short-acting anesthetic. However, development was discontinued, perhaps due to its extremely rapid elimination rate, and buthalital sodium was never marketed.

Paroxypropione, also known as paraoxypropiophenone, is a synthetic nonsteroidal estrogen which has been used medically as an antigonadotropin in Spain and Italy but appears to no longer be marketed. It was first synthesized in 1902. The antigonadotropic properties of the drug were discovered in 1951 and it entered clinical use shortly thereafter.
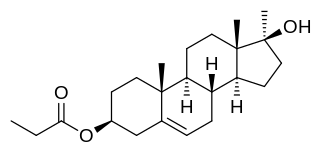
Methandriol propionate, or methylandrostenediol propionate, also known as 17α-methylandrost-5-ene-3β,17β-diol 3β-propionate, is a synthetic, injected anabolic-androgenic steroid (AAS) and a 17α-alkylated derivative of 5-androstenediol that is or was marketed by Vister in Italy. It is an androgen ester – specifically, the C3,17β propionate ester of methandriol (17α-methyl-5-androstenediol) – and acts as a prodrug of methandriol in the body. Methandriol propionate is administered by intramuscular injection and, relative to methandriol, has an extended duration via this route due to a depot effect afforded by its ester.