General anaesthetics are often defined as compounds that induce a loss of consciousness in humans or loss of righting reflex in animals. Clinical definitions are also extended to include an induced coma that causes lack of awareness to painful stimuli, sufficient to facilitate surgical applications in clinical and veterinary practice. General anaesthetics do not act as analgesics and should also not be confused with sedatives. General anaesthetics are a structurally diverse group of compounds whose mechanisms encompasses multiple biological targets involved in the control of neuronal pathways. The precise workings are the subject of some debate and ongoing research.

Anesthesia or anaesthesia is a state of controlled, temporary loss of sensation or awareness that is induced for medical or veterinary purposes. It may include some or all of analgesia, paralysis, amnesia, and unconsciousness. An individual under the effects of anesthetic drugs is referred to as being anesthetized.

Halothane, sold under the brand name Fluothane among others, is a general anaesthetic. It can be used to induce or maintain anaesthesia. One of its benefits is that it does not increase the production of saliva, which can be particularly useful in those who are difficult to intubate. It is given by inhalation.

Sevoflurane, sold under the brand name Sevorane, among others, is a sweet-smelling, nonflammable, highly fluorinated methyl isopropyl ether used as an inhalational anaesthetic for induction and maintenance of general anesthesia. After desflurane, it is the volatile anesthetic with the fastest onset. While its offset may be faster than agents other than desflurane in a few circumstances, its offset is more often similar to that of the much older agent isoflurane. While sevoflurane is only half as soluble as isoflurane in blood, the tissue blood partition coefficients of isoflurane and sevoflurane are quite similar. For example, in the muscle group: isoflurane 2.62 vs. sevoflurane 2.57. In the fat group: isoflurane 52 vs. sevoflurane 50. As a result, the longer the case, the more similar will be the emergence times for sevoflurane and isoflurane.

General anaesthesia (UK) or general anesthesia (US) is a method of medically inducing loss of consciousness that renders a patient unarousable even with painful stimuli. This effect is achieved by administering either intravenous or inhalational general anaesthetic medications, which often act in combination with an analgesic and neuromuscular blocking agent. Spontaneous ventilation is often inadequate during the procedure and intervention is often necessary to protect the airway. General anaesthesia is generally performed in an operating theater to allow surgical procedures that would otherwise be intolerably painful for a patient, or in an intensive care unit or emergency department to facilitate endotracheal intubation and mechanical ventilation in critically ill patients.

An anesthetic or anaesthetic is a drug used to induce anesthesia — in other words, to result in a temporary loss of sensation or awareness. They may be divided into two broad classes: general anesthetics, which result in a reversible loss of consciousness, and local anesthetics, which cause a reversible loss of sensation for a limited region of the body without necessarily affecting consciousness.

Enflurane is a halogenated ether. Developed by Ross Terrell in 1963, it was first used clinically in 1966. It was increasingly used for inhalational anesthesia during the 1970s and 1980s but is no longer in common use.

An inhalational anesthetic is a chemical compound possessing general anesthetic properties that can be delivered via inhalation. They are administered through a face mask, laryngeal mask airway or tracheal tube connected to an anesthetic vaporiser and an anesthetic delivery system. Agents of significant contemporary clinical interest include volatile anesthetic agents such as isoflurane, sevoflurane and desflurane, as well as certain anesthetic gases such as nitrous oxide and xenon.
Minimum alveolar concentration or MAC is the concentration, often expressed as a percentage by volume, of a vapour in the alveoli of the lungs that is needed to prevent movement in 50% of subjects in response to surgical (pain) stimulus. MAC is used to compare the strengths, or potency, of anaesthetic vapours. The concept of MAC was first introduced in 1965.

NMDA receptor antagonists are a class of drugs that work to antagonize, or inhibit the action of, the N-Methyl-D-aspartate receptor (NMDAR). They are commonly used as anesthetics for animals and humans; the state of anesthesia they induce is referred to as dissociative anesthesia.
Guedel's classification is a means of assessing of depth of general anesthesia introduced by Arthur Ernest Guedel (1883-1956) in 1920.
The Fink effect, also known as "diffusion anoxia", "diffusion hypoxia", or the "second gas effect", is a factor that influences the pO2 (partial pressure of oxygen) within the pulmonary alveoli. When water-soluble gases such as anesthetic agent N2O (nitrous oxide) are breathed in large quantities they can be dissolved in body fluids rapidly. This leads to a temporary increase in both the concentrations and partial pressures of oxygen and carbon dioxide in the alveoli.

Flurothyl (Indoklon) is a volatile liquid drug from the halogenated ether family, related to inhaled anaesthetic agents such as diethyl ether, but having the opposite effects, acting as a stimulant and convulsant. A clear and stable liquid, it has a mild ethereal odor whose vapors are non-flammable. It is excreted from the body by the lungs in an unchanged state.

Trimecaine (systematic name (2,4,6-trimethylphenylcarbamoylmethyl)diethylammonium chloride, chemical formula C15H25ClN2O) is an organic compound used as a local anesthetic and cardial antiarrhythmic. It is white crystalline powder readily soluble in water and ethanol. It is an active ingredient in products available under trademarks Mesdicain, Mesocain, Mesokain and others.
Emergence delirium is a condition in which emergence from general anesthesia is accompanied by psychomotor agitation. Some see a relation to pavor nocturnus while others see a relation to the excitement stage of anesthesia.

Fluroxene, or 2,2,2-trifluoroethyl vinyl ether, is a volatile, inhalational anesthetic, and was the first halogenated hydrocarbon anesthetic to be introduced. It was synthesized in 1951, and was introduced for clinical use in 1954, but was voluntarily withdrawn from the market in 1974 due to its potential flammability and accumulating evidence that it could cause organ toxicity. In any case, prior to being discontinued, it had largely been superseded by halothane. Fluroxene is metabolized to 2,2,2-trifluoroethanol, a compound responsible for some of the toxicity seen with fluroxene use.

Aliflurane is a halocarbon drug which was investigated as an inhalational anesthetic but was never marketed.
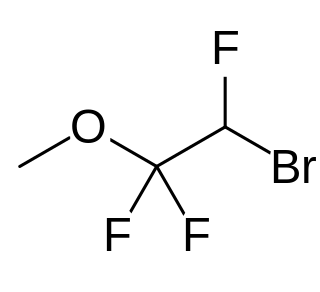
Roflurane is a halocarbon drug which was investigated as an inhalational anesthetic but was never marketed.

Teflurane is a halocarbon drug which was investigated as an inhalational anesthetic but was never marketed. Its clinical development was terminated due to a high incidence of cardiac arrhythmias in patients, similarly to the cases of halopropane and norflurane.

Halopropane is a halocarbon drug which was investigated as an inhalational anesthetic but was never marketed. Its clinical development was terminated due to a high incidence of cardiac arrhythmias in patients, similarly to the cases of teflurane and norflurane.















