
The GABAA receptor (GABAAR) is an ionotropic receptor and ligand-gated ion channel. Its endogenous ligand is γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system. Upon opening, the GABAA receptor is selectively permeable to chloride ions (Cl−) and, to a lesser extent, bicarbonate ions (HCO3−). Depending on the membrane potential and the ionic concentration difference, this can result in ionic fluxes across the pore. For instance, under physiological conditions Cl− will flow inside the cell if the membrane potential is higher than the equilibrium potential (also known as the reversal potential) for chloride ions if the receptor is activated. This causes an inhibitory effect on neurotransmission by diminishing the chance of a successful action potential occurring at the postsynaptic cell. The reversal potential of the GABAA-mediated inhibitory postsynaptic potential (IPSP) in normal solution is −70 mV, contrasting the GABAB IPSP (-100 mV).

Zaleplon, sold under the brand names Sonata among others, is a sedative-hypnotic, used to treat insomnia. It is a nonbenzodiazepine hypnotic from the pyrazolopyrimidine class.

Bretazenil (Ro16-6028) is an imidazopyrrolobenzodiazepine anxiolytic drug which is derived from the benzodiazepine family, and was invented in 1988. It is most closely related in structure to the GABA antagonist flumazenil, although its effects are somewhat different. It is classified as a high-potency benzodiazepine due to its high affinity binding to benzodiazepine binding sites where it acts as a partial agonist. Its profile as a partial agonist and preclinical trial data suggests that it may have a reduced adverse effect profile. In particular bretazenil has been proposed to cause a less strong development of tolerance and withdrawal syndrome. Bretazenil differs from traditional 1,4-benzodiazepines by being a partial agonist and because it binds to α1, α2, α3, α4, α5 and α6 subunit containing GABAA receptor benzodiazepine receptor complexes. 1,4-benzodiazepines bind only to α1, α2, α3 and α5GABAA benzodiazepine receptor complexes.

Panadiplon (U-78875) is an anxiolytic drug with a novel chemical structure that is not closely related to other drugs of this type. It has a similar pharmacological profile to the benzodiazepine family of drugs, but with mainly anxiolytic properties and relatively little sedative or amnestic effect, and so is classified as a nonbenzodiazepine anxiolytic.

L-838,417 is an anxiolytic drug used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic. The compound was developed by Merck, Sharp and Dohme.

SL651498 is an anxiolytic and anticonvulsant drug used in scientific research, with a chemical structure most closely related to β-carboline derivatives such as abecarnil and gedocarnil. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.
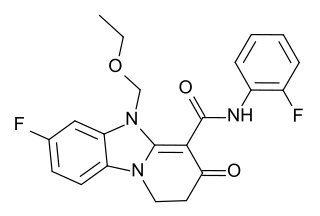
RWJ-51204 is an anxiolytic drug used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.
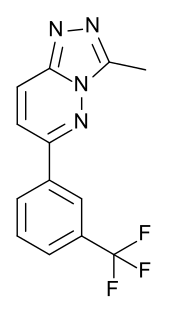
CL-218,872 is a sedative and hypnotic drug used in scientific research. It has similar effects to sedative-hypnotic benzodiazepine drugs such as triazolam, but is structurally distinct and so is classed as a nonbenzodiazepine hypnotic.

CGS-20625 is an anxiolytic drug used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic. It produces anxiolytic and anticonvulsant effects, but with no sedative effects even at high doses, and no significant muscle relaxant effects. It is orally active in humans, but with relatively low bioavailability.

Etazolate (SQ-20,009, EHT-0202) is an anxiolytic drug which is a pyrazolopyridine derivative and has unique pharmacological properties. It acts as a positive allosteric modulator of the GABAA receptor at the barbiturate binding site, as an adenosine antagonist of the A1 and A2 subtypes, and as a phosphodiesterase inhibitor selective for the PDE4 isoform. It is currently in clinical trials for the treatment of Alzheimer's disease.

Tracazolate (ICI-136,753) is an anxiolytic drug which is used in scientific research. It is a pyrazolopyridine derivative, most closely related to pyrazolopyrimidine drugs such as zaleplon, and is one of a structurally diverse group of drugs known as the nonbenzodiazepines which act at the same receptor targets as benzodiazepines but have distinct chemical structures.

SB-205384 is an anxiolytic drug. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.

ELB-139 (LS-191,811) is an anxiolytic drug with a novel chemical structure, which is used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.

TPA-023 (MK-0777) is an anxiolytic drug with a novel chemical structure, which is used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic. It is a subtype-selective, mixed allosteric modular at the benzodiazepine location on GABAA receptors, where it acts as a partial agonist at the α2 and α3 subtypes, but as a silent antagonist at α1 and α5 subtypes. It has primarily anxiolytic and anticonvulsant effects in animal tests, but with no sedative effects even at 50 times the effective anxiolytic dose.

TP-003 is an anxiolytic drug with a novel chemical structure, which is used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic. It is a positive allosteric modulator at the benzodiazepine binding site of GABAA receptors. It has modest anticonvulsant activity although less than that of diazepam.
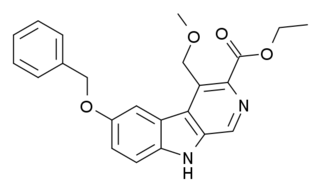
ZK-93423 is an anxiolytic drug from the β-Carboline family, closely related to abecarnil. It is a nonbenzodiazepine GABAA agonist which is not subtype selective and stimulates α1, α2, α3, and α5-subunit containing GABAA receptors equally. It has anticonvulsant, muscle relaxant and appetite stimulating properties comparable to benzodiazepine drugs. ZK-93423 has also been used as a base to develop new and improved beta-carboline derivatives and help map the binding site of the GABAA receptor.
GBLD-345 is an anxiolytic drug used in scientific research, which acts as a non-selective, full-efficacy positive allosteric modulator of the GABAA receptor. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.
Proton pump inhibitors (PPIs) block the gastric hydrogen potassium ATPase (H+/K+ ATPase) and inhibit gastric acid secretion. These drugs have emerged as the treatment of choice for acid-related diseases, including gastroesophageal reflux disease (GERD) and peptic ulcer disease. PPIs also can bind to other types of proton pumps such as those that occur in cancer cells and are finding applications in the reduction of cancer cell acid efflux and reduction of chemotherapy drug resistance.
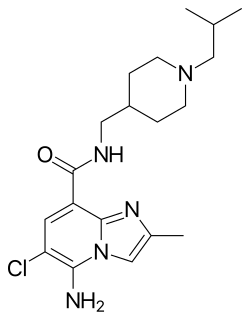
CJ-033466 is a drug which acts as a potent and selective 5-HT4 serotonin receptor partial agonist. In animal tests it stimulated gastrointestinal motility with 30 times the potency of cisapride, and with lower affinity for the hERG channel.

In pharmacology, GABAA receptor positive allosteric modulators are positive allosteric modulator (PAM) molecules that increase the activity of the GABAA receptor protein in the vertebrate central nervous system.
![Imidazo[1,2-a]pyridine--an example of imidazopyridine and a core structure of zolpidem and some compounds described below. Core structure of imidazopyridines.svg](http://upload.wikimedia.org/wikipedia/commons/thumb/9/95/Core_structure_of_imidazopyridines.svg/220px-Core_structure_of_imidazopyridines.svg.png)

















