Estrogen is a category of sex hormone responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three major endogenous estrogens that have estrogenic hormonal activity: estrone (E1), estradiol (E2), and estriol (E3). Estradiol, an estrane, is the most potent and prevalent. Another estrogen called estetrol (E4) is produced only during pregnancy.
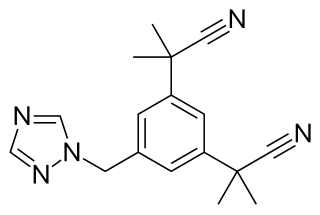
Anastrozole, sold under the brand name Arimidex among others, is an antiestrogenic medication used in addition to other treatments for breast cancer. Specifically it is used for hormone receptor-positive breast cancer. It has also been used to prevent breast cancer in those at high risk. It is taken by mouth.

Cytochromes P450 are a superfamily of enzymes containing heme as a cofactor that mostly, but not exclusively, function as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various compounds, as well as for hormone synthesis and breakdown. In 1963, Estabrook, Cooper, and Rosenthal described the role of CYP as a catalyst in steroid hormone synthesis and drug metabolism. In plants, these proteins are important for the biosynthesis of defensive compounds, fatty acids, and hormones.

Aromatase inhibitors (AIs) are a class of drugs used in the treatment of breast cancer in postmenopausal women and in men, and gynecomastia in men. They may also be used off-label to reduce estrogen conversion when supplementing testosterone exogenously. They may also be used for chemoprevention in women at high risk for breast cancer.

Estrogen receptors (ERs) are a group of proteins found inside cells. They are receptors that are activated by the hormone estrogen (17β-estradiol). Two classes of ER exist: nuclear estrogen receptors, which are members of the nuclear receptor family of intracellular receptors, and membrane estrogen receptors (mERs), which are mostly G protein-coupled receptors. This article refers to the former (ER).

Letrozole, sold under the brand name Femara among others, is an aromatase inhibitor medication that is used in the treatment of breast cancer.
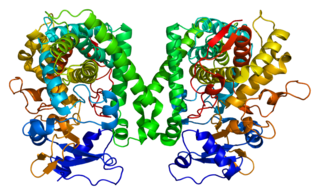
Cytochrome P4502C8 (CYP2C8) is a member of the cytochrome P450 mixed-function oxidase system involved in the metabolism of xenobiotics in the body. Cytochrome P4502C8 also possesses epoxygenase activity, i.e. it metabolizes long-chain polyunsaturated fatty acids, e.g. arachidonic acid, eicosapentaenoic acid, docosahexaenoic acid, and Linoleic acid to their biologically active epoxides.

Tibolone, sold under the brand name Livial among others, is a medication which is used in menopausal hormone therapy and in the treatment of postmenopausal osteoporosis and endometriosis. The medication is available alone and is not formulated or used in combination with other medications. It is taken by mouth.

Cytochrome P450 17A1 is an enzyme of the hydroxylase type that in humans is encoded by the CYP17A1 gene on chromosome 10. It is ubiquitously expressed in many tissues and cell types, including the zona reticularis and zona fasciculata of the adrenal cortex as well as gonadal tissues. It has both 17α-hydroxylase and 17,20-lyase activities, and is a key enzyme in the steroidogenic pathway that produces progestins, mineralocorticoids, glucocorticoids, androgens, and estrogens. More specifically, the enzyme acts upon pregnenolone and progesterone to add a hydroxyl (-OH) group at carbon 17 position (C17) of the steroid D ring, or acts upon 17α-hydroxyprogesterone and 17α-hydroxypregnenolone to split the side-chain off the steroid nucleus.

CYP27A1 is a gene encoding a cytochrome P450 oxidase, and is commonly known as sterol 27-hydroxylase. This enzyme is located in many different tissues where it is found within the mitochondria. It is most prominently involved in the biosynthesis of bile acids.

Estrogen receptor beta (ERβ) also known as NR3A2 is one of two main types of estrogen receptor—a nuclear receptor which is activated by the sex hormone estrogen. In humans ERβ is encoded by the ESR2 gene.

Progesterone receptor membrane component 1 is a protein which co-purifies with progesterone binding proteins in the liver and ovary. In humans, the PGRMC1 protein is encoded by the PGRMC1 gene.

Aromatase deficiency is a rare condition characterized by extremely low levels or complete absence of the enzyme aromatase activity in the body. It is an autosomal recessive disease resulting from various mutations of gene CYP19 (P450arom) which can lead to ambiguous genitalia and delayed puberty in females, continued linear growth into adulthood and osteoporosis in males and virilization in pregnant mothers. As of 2020, fewer than 15 cases have been identified in genetically male individuals and at least 30 cases in genetically female individuals.

Aromatase excess syndrome is a rarely diagnosed genetic and endocrine syndrome which is characterized by an overexpression of aromatase, the enzyme responsible for the biosynthesis of the estrogen sex hormones from the androgens, in turn resulting in excessive levels of circulating estrogens and, accordingly, symptoms of hyperestrogenism. It affects both sexes, manifesting itself in males as marked or complete phenotypical feminization and in females as hyperfeminization.
Steroidal aromatase inhibitors are a class of drugs that are mostly used for treating breast cancer in postmenopausal women. High levels of estrogen in breast tissue increases the risk of developing breast cancer and the enzyme aromatase is considered to be a good therapeutic target when treating breast cancer due to it being involved in the final step of estrogen biosynthetic pathway and also its inhibition will not affect production of other steroids. Aromatase Inhibitors are classified into two categories based on their structure, nonsteroidal and steroidal; the latter resemble the structure of androstenedione. Steroidal aromatase inhibitors irreversibly inhibit the enzyme by binding covalently to the binding site of aromatase so the substrate cannot access it.
Serdar Bulun is a gynecologist, with a special interest in the common gynecologic diseases, endometriosis and uterine fibroids.

The hydroxylation of estradiol is one of the major routes of metabolism of the estrogen steroid hormone estradiol. It is hydroxylated into the catechol estrogens 2-hydroxyestradiol and 4-hydroxyestradiol and into estriol (16α-hydroxyestradiol), reactions which are catalyzed by cytochrome P450 enzymes predominantly in the liver, but also in various other tissues.
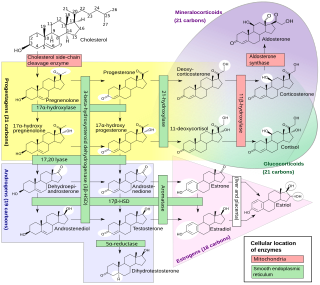
Steroidogenic enzymes are enzymes that are involved in steroidogenesis and steroid biosynthesis. They are responsible for the biosynthesis of the steroid hormones, including sex steroids and corticosteroids, as well as neurosteroids, from cholesterol. Steroidogenic enzymes are most highly expressed in classical steroidogenic tissues, such as the testis, ovary, and adrenal cortex, but are also present in other tissues in the body.

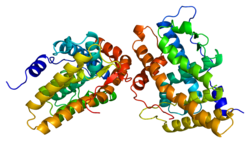
Cytochrome P450 family 19 subfamily A member 1 is a protein that in humans is encoded by the CYP19A1 gene.

Non-Steroidal Aromatase Inhibitors (NSAIs) are one of two categories of aromatase inhibitors (AIs). AIs are divided into two categories, steroidal aromatase inhibitors and non-steroidal aromatase inhibitors that is based on their mechanism of action and structure. NSAIs are mainly used to treat breast cancer in women. NSAIs binding is a reversible process where NSAIs binds to the aromatase enzyme through non-covalent interactions. When aromatase inhibitors (AIs) are used to treat breast cancer the main target is the aromatase enzyme which is responsible for the high estrogen level.