
Cytochromes P450 are a superfamily of enzymes containing heme as a cofactor that mostly, but not exclusively, function as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various compounds, as well as for hormone synthesis and breakdown, steroid hormone synthesis, drug metabolism, and the biosynthesis of defensive compounds, fatty acids, and hormones. CYP450 enzymes convert xenobiotics into hydrophilic derivatives, which are more readily excreted. In almost all of the transformations that they catalyze, P450's affect hydroxylation.

Cytochrome P450 3A4 is an important enzyme in the body, mainly found in the liver and in the intestine, which in humans is encoded by CYP3A4 gene. It oxidizes small foreign organic molecules (xenobiotics), such as toxins or drugs, so that they can be removed from the body. It is highly homologous to CYP3A5, another important CYP3A enzyme.
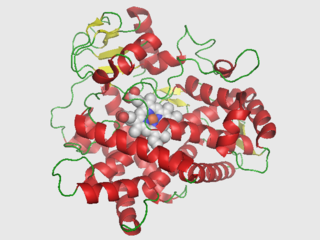
Cytochrome P450 2D6 (CYP2D6) is an enzyme that in humans is encoded by the CYP2D6 gene. CYP2D6 is primarily expressed in the liver. It is also highly expressed in areas of the central nervous system, including the substantia nigra.

Cytochrome P450 2E1 is a member of the cytochrome P450 mixed-function oxidase system, which is involved in the metabolism of xenobiotics in the body. This class of enzymes is divided up into a number of subcategories, including CYP1, CYP2, and CYP3, which as a group are largely responsible for the breakdown of foreign compounds in mammals.
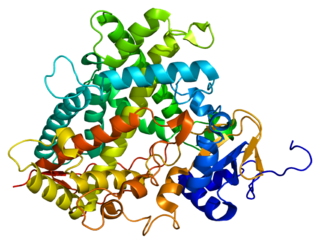
Cytochrome P450 1A2, a member of the cytochrome P450 mixed-function oxidase system, is involved in the metabolism of xenobiotics in the human body. In humans, the CYP1A2 enzyme is encoded by the CYP1A2 gene.
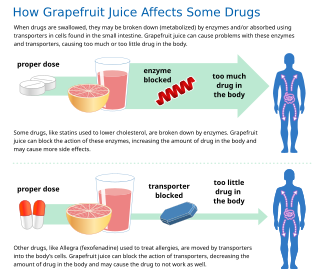
Some fruit juices and fruits can interact with numerous drugs, in many cases causing adverse effects. The effect is most studied with grapefruit and grapefruit juice, but similar effects have been observed with certain other citrus fruits.

Cytochrome P450 family 2 subfamily C member 9 is an enzyme protein. The enzyme is involved in the metabolism, by oxidation, of both xenobiotics, including drugs, and endogenous compounds, including fatty acids. In humans, the protein is encoded by the CYP2C9 gene. The gene is highly polymorphic, which affects the efficiency of the metabolism by the enzyme.

Cytochrome P450 2C19 is an enzyme protein. It is a member of the CYP2C subfamily of the cytochrome P450 mixed-function oxidase system. This subfamily includes enzymes that catalyze metabolism of xenobiotics, including some proton pump inhibitors and antiepileptic drugs. In humans, it is the CYP2C19 gene that encodes the CYP2C19 protein. CYP2C19 is a liver enzyme that acts on at least 10% of drugs in current clinical use, most notably the antiplatelet treatment clopidogrel (Plavix), drugs that treat pain associated with ulcers, such as omeprazole, antiseizure drugs such as mephenytoin, the antimalarial proguanil, and the anxiolytic diazepam.

Cytochrome P450, family 1, subfamily A, polypeptide 1 is a protein that in humans is encoded by the CYP1A1 gene. The protein is a member of the cytochrome P450 superfamily of enzymes.

Cholesterol 7 alpha-hydroxylase also known as cholesterol 7-alpha-monooxygenase or cytochrome P450 7A1 (CYP7A1) is an enzyme that in humans is encoded by the CYP7A1 gene which has an important role in cholesterol metabolism. It is a cytochrome P450 enzyme, which belongs to the oxidoreductase class, and converts cholesterol to 7-alpha-hydroxycholesterol, the first and rate limiting step in bile acid synthesis.

UGT2B7 (UDP-Glucuronosyltransferase-2B7) is a phase II metabolism isoenzyme found to be active in the liver, kidneys, epithelial cells of the lower gastrointestinal tract and also has been reported in the brain. In humans, UDP-Glucuronosyltransferase-2B7 is encoded by the UGT2B7 gene.

Aldehyde oxidase (AO) is a metabolizing enzyme, located in the cytosolic compartment of tissues in many organisms. AO catalyzes the oxidation of aldehydes into carboxylic acid, and in addition, catalyzes the hydroxylation of some heterocycles. It can also catalyze the oxidation of both cytochrome P450 and monoamine oxidase (MAO) intermediate products. AO plays an important role in the metabolism of several drugs.

Cytochrome P450 3A5 is a protein that in humans is encoded by the CYP3A5 gene.
Cytochrome P450, family 3, subfamily A, also known as CYP3A, is a human gene locus. A homologous locus is found in mice.

Cytochrome P450 4F2 is a protein that in humans is encoded by the CYP4F2 gene. This protein is an enzyme, a type of protein that catalyzes chemical reactions inside cells. This specific enzyme is part of the superfamily of cytochrome P450 (CYP) enzymes, and the encoding gene is part of a cluster of cytochrome P450 genes located on chromosome 19.

CYP2W1 is a protein that in humans is encoded by the CYP2W1 gene.

CYP2A7 is a protein that in humans is encoded by the CYP2A7 gene.

Hydroxybupropion, or 6-hydroxybupropion, is the major active metabolite of the antidepressant and smoking cessation drug bupropion. It is formed from bupropion by the liver enzyme CYP2B6 during first-pass metabolism. With oral bupropion treatment, hydroxybupropion is present in plasma at area under the curve concentrations that are as many as 16–20 times greater than those of bupropion itself, demonstrating extensive conversion of bupropion into hydroxybupropion in humans. As such, hydroxybupropion is likely to play a very important role in the effects of oral bupropion, which could accurately be thought of as functioning largely as a prodrug to hydroxybupropion. Other metabolites of bupropion besides hydroxybupropion include threohydrobupropion and erythrohydrobupropion.

Azamulin is a pleuromutilin antibiotic. As of 2021, it is not marketed in the US or Europe.

Didesmethylsibutramine is an active metabolite of the anorectic drug sibutramine that has been identified as an adulterant in weight loss supplements. Data on the activity of didesmethylsibutramine in humans is limited, although a case of psychosis associated with didesmethylsibutramine use was reported in 2019.





















