Glycosaminoglycans (GAGs) or mucopolysaccharides are long, linear polysaccharides consisting of repeating disaccharide units. The repeating two-sugar unit consists of a uronic sugar and an amino sugar, except in the case of the sulfated glycosaminoglycan keratan, where, in place of the uronic sugar there is a galactose unit. GAGs are found in vertebrates, invertebrates and bacteria. Because GAGs are highly polar molecules and attract water; the body uses them as lubricants or shock absorbers.
Cerebroside-sulfatase (EC 3.1.6.8, arylsulfatase A, cerebroside sulfate sulfatase) is an enzyme with systematic name cerebroside-3-sulfate 3-sulfohydrolase. This enzyme catalyses the following chemical reaction
Iduronidase, sold as Aldurazyme, is an enzyme with the systematic name glycosaminoglycan α-L-iduronohydrolase. It catalyses the hydrolysis of unsulfated α-L-iduronosidic linkages in dermatan sulfate.

Steroid sulfatase (STS), or steryl-sulfatase, formerly known as arylsulfatase C, is a sulfatase enzyme involved in the metabolism of steroids. It is encoded by the STS gene.

Arylsulfatase B is an enzyme associated with mucopolysaccharidosis VI.
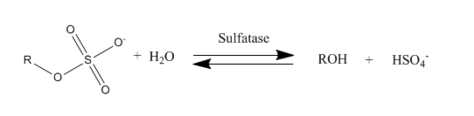
Arylsulfatase (EC 3.1.6.1, sulfatase, nitrocatechol sulfatase, phenolsulfatase, phenylsulfatase, p-nitrophenyl sulfatase, arylsulfohydrolase, 4-methylumbelliferyl sulfatase, estrogen sulfatase) is a type of sulfatase enzyme with systematic name aryl-sulfate sulfohydrolase. This enzyme catalyses the following chemical reaction

Iduronate 2-sulfatase is a sulfatase enzyme associated with Hunter syndrome. It catalyses hydrolysis of the 2-sulfate groups of the L-iduronate 2-sulfate units of dermatan sulfate, heparan sulfate and heparin.
N-acetylgalactosamine-4-sulfatase is an enzyme with systematic name N-acetyl-D-galactosamine-4-sulfate 4-sulfohydrolase. It catalyses the following reaction:

N-acetylgalactosamine-6-sulfatase is an enzyme that, in humans, is encoded by the GALNS gene.

N-acetylglucosamine-6-sulfatase (EC 3.1.6.14, glucosamine (N-acetyl)-6-sulfatase, systematic name N-acetyl-D-glucosamine-6-sulfate 6-sulfohydrolase) is an enzyme that in humans is encoded by the GNS gene. It is deficient in Sanfilippo Syndrome type IIId. It catalyses the hydrolysis of the 6-sulfate groups of the N-acetyl-D-glucosamine 6-sulfate units of heparan sulfate and keratan sulfate
In enzymology, a [heparan sulfate]-glucosamine N-sulfotransferase is an enzyme that catalyzes the chemical reaction

In enzymology, a N-sulfoglucosamine sulfohydrolase (EC 3.10.1.1), otherwise known as SGSH, is an enzyme that catalyzes the chemical reaction
The enzyme chondro-4-sulfatase (EC 3.1.6.9) catalyzes the reaction
The enzyme chondro-6-sulfatase (EC 3.1.6.10) catalyzes the reaction
The enzyme disulfoglucosamine-6-sulfatase (EC 3.1.6.1) catalyzes the reaction
In enzymology, a glucuronate-2-sulfatase is an enzyme that catalyzes the chemical reaction of cleaving off the 2-sulfate groups of the 2-O-sulfo-D-glucuronate residues of chondroitin sulfate, heparin and heparitin sulfate.
The enzyme N-acetylgalactosamine-6-sulfatase catalyzes the chemical reaction of cleaving off the 6-sulfate groups of the N-acetyl-D-galactosamine 6-sulfate units of the macromolecule chondroitin sulfate and, similarly, of the D-galactose 6-sulfate units of the macromolecule keratan sulfate.
The enzyme N-sulfoglucosamine-3-sulfatase catalyzes cleaving off the 3-sulfate groups of the N-sulfo-D-glucosamine 3-O-sulfate units of heparin.

In biochemistry, carbohydrate sulfotransferases are enzymes within the class of sulfotransferases which catalyze the transfer of the sulfate functional group to carbohydrate groups in glycoproteins and glycolipids. Carbohydrates are used by cells for a wide range of functions from structural purposes to extracellular communication. Carbohydrates are suitable for such a wide variety of functions due to the diversity in structure generated from monosaccharide composition, glycosidic linkage positions, chain branching, and covalent modification. Possible covalent modifications include acetylation, methylation, phosphorylation, and sulfation. Sulfation, performed by carbohydrate sulfotransferases, generates carbohydrate sulfate esters. These sulfate esters are only located extracellularly, whether through excretion into the extracellular matrix (ECM) or by presentation on the cell surface. As extracellular compounds, sulfated carbohydrates are mediators of intercellular communication, cellular adhesion, and ECM maintenance.
N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase is an enzyme with systematic name 3'-phosphoadenylyl-sulfate:(dermatan)-4-O-sulfo-N-acetyl-D-galactosamine 6-O-sulfotransferase. This enzyme catalyses the following chemical reaction