External links
- Exodeoxyribonucleases at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
| Activity | |
|---|---|
| Regulation | |
| Classification | |
| Kinetics | |
| Types |
|
Exodeoxyribonucleases are both exonucleases and deoxyribonucleases. They catalyze digestion of the ends of linear DNA. They are a type of esterase. They are classified EC 3.1.11.
Deoxyribonuclease refers to a group of glycoprotein endonucleases which are enzymes that catalyze the hydrolytic cleavage of phosphodiester linkages in the DNA backbone, thus degrading DNA. The role of the DNase enzyme in cells includes breaking down extracellular DNA (ecDNA) excreted by apoptosis, necrosis, and neutrophil extracellular traps (NET) of cells to help reduce inflammatory responses that otherwise are elicited. A wide variety of deoxyribonucleases are known and fall into one of two families, which differ in their substrate specificities, chemical mechanisms, and biological functions. Laboratory applications of DNase include purifying proteins when extracted from prokaryotic organisms. Additionally, DNase has been applied as a treatment for diseases that are caused by ecDNA in the blood plasma. Assays of DNase are emerging in the research field as well.

Exodeoxyribonuclease V is an enzyme of E. coli that initiates recombinational repair from potentially lethal double strand breaks in DNA which may result from ionizing radiation, replication errors, endonucleases, oxidative damage, and a host of other factors. The RecBCD enzyme is both a helicase that unwinds, or separates the strands of DNA, and a nuclease that makes single-stranded nicks in DNA. It catalyses exonucleolytic cleavage in either 5′- to 3′- or 3′- to 5′-direction to yield 5′-phosphooligonucleotides.

Stanford Moore was an American biochemist. He shared a Nobel Prize in Chemistry in 1972, with Christian B. Anfinsen and William Howard Stein, for work done at Rockefeller University on the structure of the enzyme ribonuclease and for contributing to the understanding of the connection between the chemical structure and catalytic activity of the ribonuclease molecule.
In biochemistry, an esterase is a class of enzyme that splits esters into an acid and an alcohol in a chemical reaction with water called hydrolysis.
Type I site-specific deoxyribonuclease is an enzyme. This enzyme catalyses the following chemical reaction

Deoxyribonuclease I, is an endonuclease of the DNase family coded by the human gene DNASE1. DNase I is a nuclease that cleaves DNA preferentially at phosphodiester linkages adjacent to a pyrimidine nucleotide, yielding 5'-phosphate-terminated polynucleotides with a free hydroxyl group on position 3', on average producing tetranucleotides. It acts on single-stranded DNA, double-stranded DNA, and chromatin. In addition to its role as a waste-management endonuclease, it has been suggested to be one of the deoxyribonucleases responsible for DNA fragmentation during apoptosis.
Deoxyribonuclease II is an endonuclease that hydrolyzes phosphodiester linkages of deoxyribonucleotide in native and denatured DNA, yielding products with 3'-phosphates and 5'-hydroxyl ends, which occurs as a result of single-strand cleaving mechanism. As the name implies, it functions optimally at acid pH because it is commonly found in low pH environment of lysosomes.
Deoxyribonuclease V is an enzyme. This enzyme catalyses the following chemical reaction
Exodeoxyribonuclease I is an enzyme that catalyses the following chemical reaction:

An exoribonuclease is an exonuclease ribonuclease, which are enzymes that degrade RNA by removing terminal nucleotides from either the 5' end or the 3' end of the RNA molecule. Enzymes that remove nucleotides from the 5' end are called 5'-3' exoribonucleases, and enzymes that remove nucleotides from the 3' end are called 3'-5' exoribonucleases.
In biochemistry, an endodeoxyribonuclease is a class of enzyme which is a type of deoxyribonuclease, itself a type of endonuclease. They catalyze cleavage of the phosphodiester bonds in DNA. They are classified with EC numbers 3.1.21 through 3.1.25.
Deoxyribonuclease IV (phage-T4-induced) is catalyzes the degradation nucleotides in DsDNA by attacking the 5'-terminal end.

Deoxyribonuclease-1-like 2 is an enzyme that in humans is encoded by the DNASE1L2 gene.

Syncephalastrum racemosum is a filamentous fungus.
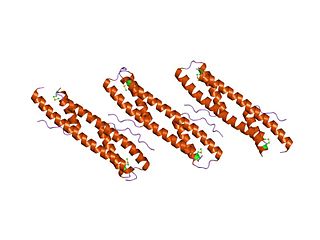
The enzyme exodeoxyribonuclease VII is a bacterial exonuclease enzyme. It is composed of two nonidentical subunits; one large subunit and 4 small ones. that catalyses exonucleolytic cleavage in either 5′- to 3′- or 3′- to 5′-direction to yield nucleoside 5′-phosphates. The large subunit also contains an N-terminal OB-fold domain that binds to nucleic acids.
Exodeoxyribonuclease III is an enzyme that catalyses the following reaction
Exodeoxyribonuclease (lambda-induced) is an exonuclease. This enzyme catalyses the following chemical reaction
Aspergillus deoxyribonuclease K1 is an enzyme. This enzyme catalyses the following chemical reaction
Deoxyribonuclease X is an enzyme. This enzyme catalyses the following chemical reaction