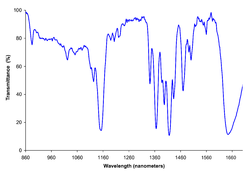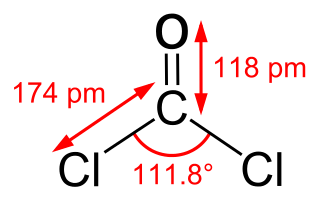
Phosgene is an organic chemical compound with the formula COCl2. It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. It can be thought of chemically as the double acyl chloride analog of carbonic acid, or structurally as formaldehyde with the hydrogen atoms replaced by chlorine atoms. Phosgene is a valued and important industrial building block, especially for the production of precursors of polyurethanes and polycarbonate plastics.

In organic chemistry, isocyanate is the functional group with the formula R−N=C=O. Organic compounds that contain an isocyanate group are referred to as isocyanates. An organic compound with two isocyanate groups is known as a diisocyanate. Diisocyanates are manufactured for the production of polyurethanes, a class of polymers.

The haloalkanes are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen.
Chloroform, or trichloromethane, is an organic compound with the formula CHCl3 and a common solvent. It is a very volatile, colorless, strong-smelling, dense liquid produced on a large scale as a precursor to refrigerants and PTFE. Chloroform is a trihalomethane that serves as a powerful anesthetic, euphoriant, anxiolytic, and sedative when inhaled or ingested. Chloroform was used as an anesthetic between the 19th century and the first half of the 20th century. It is miscible with many solvents but it is only very slightly soluble in water.

Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent. It is an isomer of another solvent, butanone.

Tetrachloroethylene, also known under the systematic name tetrachloroethene, or perchloroethylene, and abbreviations such as "perc", and "PCE", is a chlorocarbon with the formula Cl2C=CCl2. It is a colorless liquid widely used for dry cleaning of fabrics, hence it is sometimes called "dry-cleaning fluid". It also has its uses as an effective automotive brake cleaner. It has a mild sweet, sharp odor, detectable by most people at a concentration of 50 ppm.
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula CH3Cl. One of the haloalkanes, it is a colorless, sweet-smelling, flammable gas. Methyl chloride is a crucial reagent in industrial chemistry, although it is rarely present in consumer products, and was formerly utilized as a refrigerant. Most chloromethane is biogenic.

The organic compound 1,1,1-trichloroethane, also known as methyl chloroform and chlorothene, is a chloroalkane with the chemical formula CH3CCl3. It is an isomer of 1,1,2-trichloroethane. This colorless, sweet-smelling liquid was once produced industrially in large quantities for use as a solvent. It is regulated by the Montreal Protocol as an ozone-depleting substance and its use is being rapidly phased out.

Hydrogen bromide is the inorganic compound with the formula HBr. It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temperature. Aqueous solutions that are 47.6% HBr by mass form a constant-boiling azeotrope mixture that boils at 124.3 °C (255.7 °F). Boiling less concentrated solutions releases H2O until the constant-boiling mixture composition is reached.

Methylamine is an organic compound with a formula of CH3NH2. This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine.
Morpholine is an organic chemical compound having the chemical formula O(CH2CH2)2NH. This heterocycle features both amine and ether functional groups. Because of the amine, morpholine is a base; its conjugate acid is called morpholinium. For example, treating morpholine with hydrochloric acid makes the salt morpholinium chloride. It is a colorless liquid with a weak, ammonia- or fish-like odor. The naming of morpholine is attributed to Ludwig Knorr, who incorrectly believed it to be part of the structure of morphine.
Trichlorofluoromethane, also called freon-11, CFC-11, or R-11, is a chlorofluorocarbon (CFC). It is a colorless, faintly ethereal, and sweetish-smelling liquid that boils around room temperature. CFC-11 is a Class 1 ozone-depleting substance which damages Earth's protective stratospheric ozone layer.

tert-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as t-BuOH). Its isomers are 1-butanol, isobutanol, and butan-2-ol. tert-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor. It is miscible with water, ethanol and diethyl ether.

Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols, it is a water-soluble, colourless liquid. It is more toxic than typical small alcohols. Allyl alcohol is used as a precursor to many specialized compounds such as flame-resistant materials, drying oils, and plasticizers. Allyl alcohol is the smallest representative of the allylic alcohols.
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colorless liquid is a reactive organochlorine compound that is a widely used chemical building block.

Dimethoxymethane, also called methylal, is a colorless flammable liquid with a low boiling point, low viscosity and excellent dissolving power. It has a chloroform-like odor and a pungent taste. It is the dimethyl acetal of formaldehyde. Dimethoxymethane is soluble in three parts water and miscible with most common organic solvents.

Bromochloromethane or methylene bromochloride and Halon 1011 is a mixed halomethane. It is a heavy low-viscosity liquid with refractive index 1.4808.

1-Bromopropane (n-propylbromide or nPB) is an organobromine compound with the chemical formula CH3CH2CH2Br. It is a colorless liquid that is used as a solvent. It has a characteristic hydrocarbon odor. Its industrial applications increased dramatically in the 21st century due to the phasing out of chlorofluorocarbons and chloroalkanes such as 1,1,1-Trichloroethane under the Montreal Protocol.

Chloroacetyl chloride is a chlorinated acyl chloride. It is a bifunctional compound, making it a useful building block chemical.

Perchloromethyl mercaptan is the organosulfur compound with the formula CCl3SCl. It is mainly used as an intermediate for the synthesis of dyes and fungicides (captan, folpet). It is a colorless oil, although commercial samples are yellowish. It is insoluble in water but soluble in organic solvents. It has a foul, unbearable, acrid odor. Perchloromethyl mercaptan is the original name. The systematic name is trichloromethanesulfenyl chloride, because the compound is a sulfenyl chloride, not a mercaptan.