Pentane is an organic compound with the formula C5H12—that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer, in which case pentanes refers to a mixture of them; the other two are called isopentane (methylbutane) and neopentane (dimethylpropane). Cyclopentane is not an isomer of pentane because it has only 10 hydrogen atoms where pentane has 12.

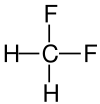
Fluoromethane, also known as methyl fluoride, Freon 41, Halocarbon-41 and HFC-41, is a non-toxic, liquefiable, and flammable gas at standard temperature and pressure. It is made of carbon, hydrogen, and fluorine. The name stems from the fact that it is methane (CH4) with a fluorine atom substituted for one of the hydrogen atoms. It is used in semiconductor manufacturing processes as an etching gas in plasma etch reactors.
Cyclopentane (also called C pentane) is a highly flammable alicyclic hydrocarbon with chemical formula C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It occurs as a colorless liquid with a petrol-like odor. Its freezing point is −94 °C and its boiling point is 49 °C. Cyclopentane is in the class of cycloalkanes, being alkanes that have one or more carbon rings. It is formed by cracking cyclohexane in the presence of alumina at a high temperature and pressure.
But-2-ene is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting cis/trans-isomerism (also known as (E/Z)-isomerism); that is, it exists as two geometric isomers cis-but-2-ene ((Z)-but-2-ene) and trans-but-2-ene ((E)-but-2-ene).

Propyl acetate, also known as propyl ethanoate, is an organic compound. Nearly 20,000 tons are produced annually for use as a solvent. This colorless liquid is known by its characteristic odor of pears. Due to this fact, it is commonly used in fragrances and as a flavor additive. It is formed by the esterification of acetic acid and propan-1-ol, often via Fischer–Speier esterification, with sulfuric acid as a catalyst and water produced as a byproduct.

1-Nonanol is a straight chain fatty alcohol with nine carbon atoms and the molecular formula CH3(CH2)8OH. It is a colorless oily liquid with a citrus odor similar to citronella oil.
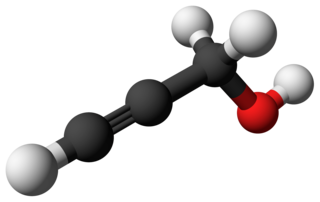
Propargyl alcohol, or 2-propyn-1-ol, is an organic compound with the formula C3H4O. It is the simplest stable alcohol containing an alkyne functional group. Propargyl alcohol is a colorless viscous liquid that is miscible with water and most polar organic solvents.
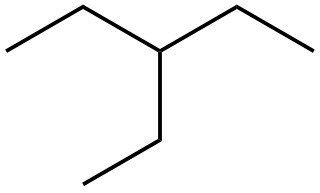
3-Ethylpentane (C7H16) is a branched saturated hydrocarbon. It is an alkane, and one of the many structural isomers of heptane, consisting of a five carbon chain with a two carbon branch at the middle carbon.
An occupational exposure limit is an upper limit on the acceptable concentration of a hazardous substance in workplace air for a particular material or class of materials. It is typically set by competent national authorities and enforced by legislation to protect occupational safety and health. It is an important tool in risk assessment and in the management of activities involving handling of dangerous substances. There are many dangerous substances for which there are no formal occupational exposure limits. In these cases, hazard banding or control banding strategies can be used to ensure safe handling.
The Institute for Occupational Safety and Health of the German Social Accident Insurance is a German institute located in Sankt Augustin near Bonn and is a main department of the German Social Accident Insurance. Belonging to the Statutory Accident Insurance means that IFA is a non-profit institution.
The monochlorophenols are chemical compounds consisting of phenol substituted with a chlorine atom. There are three isomers, 2-chlorophenol, 3-chlorophenol, and 4-chlorophenol.
Arsenic pentafluoride is a chemical compound of arsenic and fluorine. It is a toxic, colorless gas. The oxidation state of arsenic is +5.
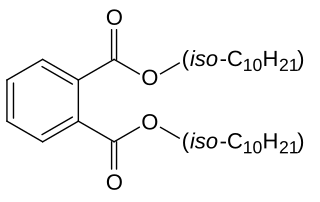
Diisodecyl phthalate (DIDP) is a commonly used plasticizer used in the production of plastic and plastic coating to increase flexibility. It is a mixture of compounds derived from the esterification of phthalic acid and isomeric decyl alcohols.
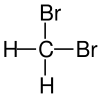
The monohalomethanes are organic compounds in which a hydrogen atom in methane is replaced by a halogen. They belong to the haloalkanes or to the subgroup of halomethanes.
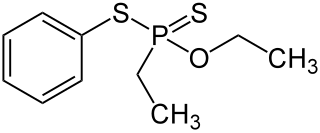
Fonofos is an organothiophosphate insecticide primarily used on corn. It is highly toxic and listed as an extremely hazardous substance.

Oxydemeton-methyl is an organothiophosphate insecticide. It is primarily used to control aphids, mites, and thrips.
Dinitrobenzenes are nitrobenzenes composed of a benzene ring and two nitro group (-NO2) substituents. The three possible arrangements of the nitro groups afford three isomers, 1,2-dinitrobenzene, 1,3-dinitrobenzene, and 1,4-dinitrobenzene. Each isomer has the chemical formula C6H4N2O4 and a molar mass of about 168.11 g/mol. 1,3-Dinitrobenzene is the most common isomer and it is used in the manufacture of explosives.
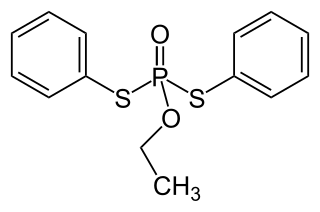
Edifenphos is a systemic fungicide that inhibits phosphatidylcholine biosynthesis. It was introduced in 1966 by Bayer to combat blast fungus and Pellicularia sasakii in rice cultivation. It was never authorized for use in the EU.
GESTIS Substance Database is a freely accessible online information system on chemical compounds. It is maintained by the Institut für Arbeitsschutz der Deutschen Gesetzlichen Unfallversicherung. Information on occupational medicine and first aid is compiled by Henning Heberer and his team.
1-Octanethiol, also called 1-mercaptooctane, is an organic compound.