Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixture and the condensation of the vapors in a still.

Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. A high concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humidity affects rate of evaporation of water. When the molecules of the liquid collide, they transfer energy to each other based on how they collide. When a molecule near the surface absorbs enough energy to overcome the vapor pressure, it will escape and enter the surrounding air as a gas. When evaporation occurs, the energy removed from the vaporized liquid will reduce the temperature of the liquid, resulting in evaporative cooling.
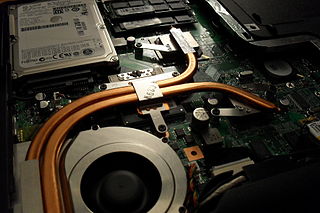
A heat pipe is a heat-transfer device that employs phase transition to transfer heat between two solid interfaces.

An evaporative cooler is a device that cools air through the evaporation of water. Evaporative cooling differs from other air conditioning systems, which use vapor-compression or absorption refrigeration cycles. Evaporative cooling exploits the fact that water will absorb a relatively large amount of heat in order to evaporate. The temperature of dry air can be dropped significantly through the phase transition of liquid water to water vapor (evaporation). This can cool air using much less energy than refrigeration. In extremely dry climates, evaporative cooling of air has the added benefit of conditioning the air with more moisture for the comfort of building occupants.

A chiller is a machine that removes heat from a liquid coolant via a vapor-compression, adsorption refrigeration, or absorption refrigeration cycles. This liquid can then be circulated through a heat exchanger to cool equipment, or another process stream. As a necessary by-product, refrigeration creates waste heat that must be exhausted to ambience, or for greater efficiency, recovered for heating purposes. Vapor compression chillers may use any of a number of different types of compressors. Most common today are the hermetic scroll, semi-hermetic screw, or centrifugal compressors. The condensing side of the chiller can be either air or water cooled. Even when liquid cooled, the chiller is often cooled by an induced or forced draft cooling tower. Absorption and adsorption chillers require a heat source to function.

Psychrometrics is the field of engineering concerned with the physical and thermodynamic properties of gas-vapor mixtures.

An absorption refrigerator is a refrigerator that uses a heat source to provide the energy needed to drive the cooling process. Solar energy, burning a fossil fuel, waste heat from factories, and district heating systems are examples of convenient heat sources that can be used. An absorption refrigerator uses two coolants: the first coolant performs evaporative cooling and then is absorbed into the second coolant; heat is needed to reset the two coolants to their initial states. Absorption refrigerators are commonly used in recreational vehicles (RVs), campers, and caravans because the heat required to power them can be provided by a propane fuel burner, by a low-voltage DC electric heater or by a mains-powered electric heater. Absorption refrigerators can also be used to air-condition buildings using the waste heat from a gas turbine or water heater in the building. Using waste heat from a gas turbine makes the turbine very efficient because it first produces electricity, then hot water, and finally, air-conditioning—trigeneration.

This timeline of heat engine technology describes how heat engines have been known since antiquity but have been made into increasingly useful devices since the 17th century as a better understanding of the processes involved was gained. A heat engine is any system that converts heat to mechanical energy, which can then be used to do mechanical work.They continue to be developed today.
Economizers, or economisers (UK), are mechanical devices intended to reduce energy consumption, or to perform useful function such as preheating a fluid. The term economizer is used for other purposes as well. Boiler, power plant, heating, refrigeration, ventilating, and air conditioning (HVAC) uses are discussed in this article. In simple terms, an economizer is a heat exchanger.

Vapour-compression refrigeration or vapor-compression refrigeration system (VCRS), in which the refrigerant undergoes phase changes, is one of the many refrigeration cycles and is the most widely used method for air conditioning of buildings and automobiles. It is also used in domestic and commercial refrigerators, large-scale warehouses for chilled or frozen storage of foods and meats, refrigerated trucks and railroad cars, and a host of other commercial and industrial services. Oil refineries, petrochemical and chemical processing plants, and natural gas processing plants are among the many types of industrial plants that often utilize large vapor-compression refrigeration systems. Cascade refrigeration systems may also be implemented using two compressors.

A turboexpander, also referred to as a turbo-expander or an expansion turbine, is a centrifugal or axial-flow turbine, through which a high-pressure gas is expanded to produce work that is often used to drive a compressor or generator.
A loop heat pipe (LHP) is a two-phase heat transfer device that uses capillary action to remove heat from a source and passively move it to a condenser or radiator. LHPs are similar to heat pipes but have the advantage of being able to provide reliable operation over long distance and the ability to operate against gravity. They can transport a large heat load over a long distance with a small temperature difference. Different designs of LHPs ranging from powerful, large size LHPs to miniature LHPs have been developed and successfully employed in a wide sphere of applications both ground and space-based applications.

A thermal expansion valve or thermostatic expansion valve is a component in vapor-compression refrigeration and air conditioning systems that controls the amount of refrigerant released into the evaporator and is intended to regulate the superheat of the refrigerant that flows out of the evaporator to a steady value. Although often described as a "thermostatic" valve, an expansion valve is not able to regulate the evaporator's temperature to a precise value. The evaporator's temperature will vary only with the evaporating pressure, which will have to be regulated through other means.

An evaporator is a type of heat exchanger device that facilitates evaporation by utilizing conductive and convective heat transfer, which provides the necessary thermal energy for phase transition from liquid to vapor. Within evaporators, a circulating liquid is exposed to an atmospheric or reduced pressure environment, causing it to boil at a lower temperature compared to normal atmospheric boiling.
A cryophorus is a glass container containing liquid water and water vapor. It is used in physics courses to demonstrate rapid freezing by evaporation. A typical cryophorus has a bulb at one end connected to a tube of the same material. When the liquid water is manipulated into the bulbed end and the other end is submerged into a freezing mixture, the gas pressure drops as it is cooled. The liquid water begins to evaporate, producing more water vapor. Evaporation causes the water to cool rapidly to its freezing point and it solidifies suddenly.
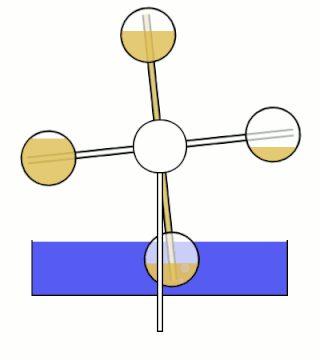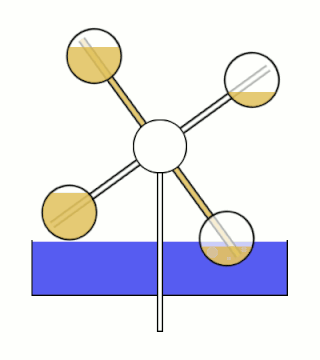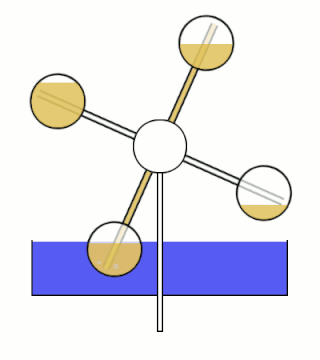
The Minto wheel is a heat engine named after Wally Minto. The engine consists of a set of sealed chambers arranged in a circle, with each chamber connected to the chamber opposite it. One chamber in each connected pair is filled with a liquid with a low boiling point. Ideally, the working fluid also has a high vapor pressure and density.

In chemistry, a condenser is laboratory apparatus used to condense vapors – that is, turn them into liquids – by cooling them down.

The alcohol thermometer or spirit thermometer has a similar construction and theory of operation as a mercury-in-glass thermometer. However, the thermometric fluid of an alcohol thermometer is less toxic and evaporates quickly making it a safer alternative to mercury thermometers. The ethanol version is the most widely used due to the low cost and relatively low hazard posed by the liquid in case of breakage.

Steam is a substance containing water in the gas phase, often mixed with air and/or an aerosol of liquid water droplets. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization. Steam that is saturated or superheated is invisible; however, wet steam, a visible mist or aerosol of water droplets, is often referred to as "steam".

A hand boiler or love meter is a glass sculpture used as an experimental tool to demonstrate vapour-liquid equilibrium, or as a collector's item to whimsically "measure love." It consists of a lower bulb containing a volatile liquid and a mixture of gases that is connected usually by a twisting glass tube that connects to an upper or "receiving" glass bulb.