
Nitrazepam, sold under the brand name Mogadon among others, is a hypnotic drug of the benzodiazepine class used for short-term relief from severe, disabling anxiety and insomnia. It also has sedative (calming) properties, as well as amnestic, anticonvulsant, and skeletal muscle relaxant effects.

Prazepam is a benzodiazepine derivative drug developed by Warner-Lambert in the 1960s. It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties. Prazepam is a prodrug for desmethyldiazepam which is responsible for the therapeutic effects of prazepam.

Camazepam is a benzodiazepine psychoactive drug, marketed under the brand names Albego, Limpidon and Paxor. It is the dimethyl carbamate ester of temazepam, a metabolite of diazepam. While it possesses anxiolytic, anticonvulsant, skeletal muscle relaxant and hypnotic properties it differs from other benzodiazepines in that its anxiolytic properties are particularly prominent but has comparatively limited anticonvulsant, hypnotic and skeletal muscle relaxant properties.
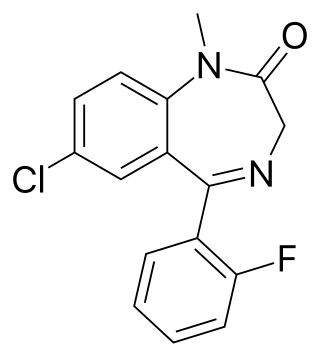
Fludiazepam, marketed under the brand name Erispan (エリスパン) is a potent benzodiazepine and 2ʹ-fluoro derivative of diazepam, originally developed by Hoffmann-La Roche in the 1960s. It is marketed in Japan and Taiwan. It exerts its pharmacological properties via enhancement of GABAergic inhibition. Fludiazepam has 4 times more binding affinity for benzodiazepine receptors than diazepam. It possesses anxiolytic, anticonvulsant, sedative, hypnotic and skeletal muscle relaxant properties. Fludiazepam has been used recreationally.

Rilmazafone is a water-soluble prodrug developed in Japan. Once metabolized, rilmazafone is converted into several benzodiazepine metabolites that have sedative and hypnotic effects. These metabolites induce impairment of motor function and have hypnotic properties.

Tifluadom is a benzodiazepine derivative with an unusual activity profile. Unlike most benzodiazepines, tifluadom has no activity at the GABAA receptor, but instead is a selective agonist for the κ-opioid receptor. It has potent analgesic and diuretic effects in animals, and also has sedative effects and stimulates appetite.

Sulazepam is a benzodiazepine derivative. It is the thioamide derivative of diazepam. It is metabolised into diazepam, desmethyldiazepam and oxydiazepam. It has sedative, muscle relaxant, hypnotic, anticonvulsant and anxiolytic properties like those of other benzodiazepines. It was never marketed.

Meclonazepam ((S)-3-methylclonazepam) was discovered by a team at Hoffmann-La Roche in the 1970s and is a drug which is a benzodiazepine derivative similar in structure to clonazepam. It has sedative and anxiolytic actions like those of other benzodiazepines, and also has anti-parasitic effects against the parasitic worm Schistosoma mansoni.

Pyrazolam (SH-I-04) is a benzodiazepine derivative originally developed by a team led by Leo Sternbach at Hoffman-La Roche in the 1970s. It has since been "rediscovered" and sold as a designer drug since 2012.

Flubromazepam is a benzodiazepine derivative which was first synthesized in 1960, but was never marketed and did not receive any further attention or study until late 2012 when it appeared on the grey market as a novel designer drug.

3-Hydroxyphenazepam is a benzodiazepine with hypnotic, sedative, anxiolytic, and anticonvulsant properties. It is an active metabolite of phenazepam, as well as the active metabolite of the benzodiazepine prodrug cinazepam. Relative to phenazepam, 3-hydroxyphenazepam has diminished myorelaxant properties, but is about equivalent in most other regards. Like other benzodiazepines, 3-hydroxyphenazepam behaves as a positive allosteric modulator of the benzodiazepine site of the GABAA receptor with an EC50 value of 10.3 nM. It has been sold online as a designer drug.
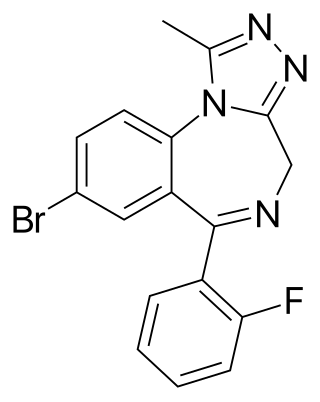
Flubromazolam (JYI-73) is a triazolobenzodiazepine (TBZD), which are benzodiazepine (BZD) derivatives. Flubromazolam is reputed to be highly potent, and concerns have been raised that clonazolam and flubromazolam in particular may pose comparatively higher risks than other designer benzodiazepines, due to their ability to produce strong sedation and amnesia at oral doses of as little as 0.5 mg. Life-threatening adverse reactions have been observed at doses of only 3 mg of flubromazolam.

Nitrazolam is a triazolobenzodiazepine (TBZD) , which are benzodiazepine (BZD) derivatives, that has been sold online as a designer drug.

Fluclotizolam is a thienotriazolodiazepine derivative which was first synthesised in 1979, but was never marketed. It has subsequently been sold as a designer drug, first being definitively identified in 2017.

Ro20-8552 is a benzodiazepine derivative with sedative and anxiolytic effects, which has been sold as a designer drug.

Fluadinazolam is a benzodiazepine derivative developed in 1973, with sedative and anxiolytic effects. It is a derivative of the never commercially marketed benzodiazepine adinazolam and has similarly been sold as a designer drug.
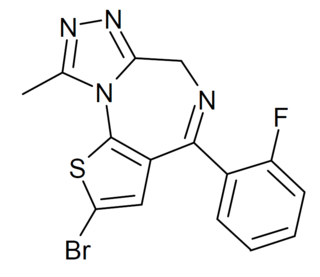
Flubrotizolam is a thienotriazolodiazepine derivative with potent sedative and anxiolytic effects, which has been sold as a designer drug.

Ro20-8065 (8-Chloronorflurazepam) is a benzodiazepine derivative with anticonvulsant and muscle relaxant effects, which has been sold as a designer drug.

Ro07-5220 (6'-Chlorodiclazepam) is a benzodiazepine derivative with sedative, anxiolytic, anticonvulsant and muscle relaxant effects, which has been sold as a designer drug.



















