
Bupropion, sold under the brand name Wellbutrin among others, is an atypical antidepressant primarily used to treat major depressive disorder and to support smoking cessation. It is also popular as an add-on medication in the cases of "incomplete response" to the first-line selective serotonin reuptake inhibitor (SSRI) antidepressant. Bupropion has several features that distinguish it from other antidepressants: it does not usually cause sexual dysfunction, it is not associated with weight gain and sleepiness, and it is more effective than SSRIs at improving symptoms of hypersomnia and fatigue. Bupropion, particularly the immediate release formulation, carries a higher risk of seizure than many other antidepressants, hence caution is recommended in patients with a history of seizure disorder.

Omeprazole, sold under the brand names Prilosec and Losec, among others, is a medication used in the treatment of gastroesophageal reflux disease (GERD), peptic ulcer disease, and Zollinger–Ellison syndrome. It is also used to prevent upper gastrointestinal bleeding in people who are at high risk. Omeprazole is a proton-pump inhibitor (PPI) and its effectiveness is similar to that of other PPIs. It can be taken by mouth or by injection into a vein. It is also available in the fixed-dose combination medication omeprazole/sodium bicarbonate as Zegerid and as Konvomep.
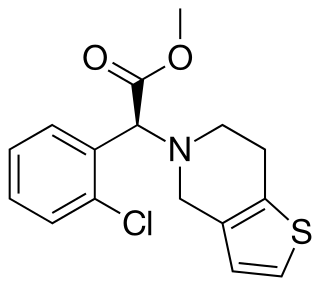
Clopidogrel—sold under the brand names Plavix and Deplat, among others—is an antiplatelet medication used to reduce the risk of heart disease and stroke in those at high risk. It is also used together with aspirin in heart attacks and following the placement of a coronary artery stent. It is taken by mouth. Its effect starts about two hours after intake and lasts for five days.
An anorectic or anorexic is a drug which reduces appetite, resulting in lower food consumption, leading to weight loss. By contrast, an appetite stimulant is referred to as orexigenic.
A dopamine reuptake inhibitor (DRI) is a class of drug which acts as a reuptake inhibitor of the monoamine neurotransmitter dopamine by blocking the action of the dopamine transporter (DAT). Reuptake inhibition is achieved when extracellular dopamine not absorbed by the postsynaptic neuron is blocked from re-entering the presynaptic neuron. This results in increased extracellular concentrations of dopamine and increase in dopaminergic neurotransmission.

Sibutramine, formerly sold under the brand name Meridia among others, is an appetite suppressant which has been discontinued in many countries. It works as a serotonin–norepinephrine reuptake inhibitor similar to a tricyclic antidepressant. Until 2010, it was widely marketed and prescribed as an adjunct in the treatment of obesity along with diet and exercise. It has been associated with increased cardiovascular diseases and strokes and has been withdrawn from the market in 2010 in several countries and regions including Australia, Canada, China, the European Union, Hong Kong, India, Mexico, New Zealand, the Philippines, Thailand, the United Kingdom, and the United States. However, the drug remains available in some countries.
A serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI), also known as a triple reuptake inhibitor (TRI), is a type of drug that acts as a combined reuptake inhibitor of the monoamine neurotransmitters serotonin, norepinephrine, and dopamine. It does this by concomitantly inhibiting the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT), respectively. Inhibition of the reuptake of these neurotransmitters increases their extracellular concentrations and, therefore, results in an increase in serotonergic, adrenergic, and dopaminergic neurotransmission. The naturally-occurring and potent SNDRI cocaine is widely used recreationally and often illegally for the euphoric effects it produces.

Desmetramadol (INN), also known as O-desmethyltramadol (O-DSMT), is an opioid analgesic and the main active metabolite of tramadol. Tramadol is demethylated by the liver enzyme CYP2D6 to desmetramadol in the same way as codeine, and so similarly to the variation in effects seen with codeine, individuals who have a less active form of CYP2D6 will tend to have reduced analgesic effects from tramadol. Because desmetramadol itself does not need to be metabolized to induce an analgesic effect, it can be used in individuals with low CYP2D6 activity unlike tramadol.

Tesofensine (NS2330) is a serotonin–noradrenaline–dopamine reuptake inhibitor from the phenyltropane family of drugs, which is being developed for the treatment of obesity. Tesofensine was originally developed by a Danish biotechnology company, NeuroSearch, who transferred the rights to Saniona in 2014.

Seproxetine, also known as (S)-norfluoxetine, is a selective serotonin reuptake inhibitor (SSRI). It is the S enantiomer of norfluoxetine, the main active metabolite of the widely used antidepressant fluoxetine; it is nearly 4 times more selective for stimulating neurosteroid synthesis relative to serotonin reuptake inhibition than fluoxetine. It is formed through the demethylation, or removal of a methyl group, of norfluoxetine. Seproxetine is both an inhibitor of serotonin and dopamine transporters, 5-HT2A and 5-HT2C receptors. It was being investigated by Eli Lilly and Company as an antidepressant; however, it inhibited the KvLQT1 protein, which is responsible for the management of the QT interval. This is the time it takes for the heart to contract and recover. Due to the inhibition, the QT interval was prolonged, which could lead to significant cardiac side complications. Due to this, development of the medication was discontinued. Tests on its efficacy found that it was equivalent to fluoxetine, but sixteen times more powerful than the R enantiomer of norfluoxetine.
Adenosine diphosphate (ADP) receptor inhibitors are a drug class of antiplatelet agents, used in the treatment of acute coronary syndrome (ACS) or in preventive treatment for patients who are in risk of thromboembolism, myocardial infarction or a stroke. These drugs antagonize the P2Y12 platelet receptors and therefore prevent the binding of ADP to the P2Y12 receptor. This leads to a decrease in aggregation of platelets, prohibiting thrombus formation. The P2Y12 receptor is a surface bound protein found on blood platelets. They belong to G protein-coupled purinergic receptors (GPCR) and are chemoreceptors for ADP.

A serotonin–dopamine reuptake inhibitor (SDRI) is a type of drug which acts as a reuptake inhibitor of the monoamine neurotransmitters serotonin and dopamine by blocking the actions of the serotonin transporter (SERT) and dopamine transporter (DAT), respectively. This in turn leads to increased extracellular concentrations of serotonin and dopamine, and, therefore, an increase in serotonergic and dopaminergic neurotransmission.
A monoamine reuptake inhibitor (MRI) is a drug that acts as a reuptake inhibitor of one or more of the three major monoamine neurotransmitters serotonin, norepinephrine, and dopamine by blocking the action of one or more of the respective monoamine transporters (MATs), which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT). This in turn results in an increase in the synaptic concentrations of one or more of these neurotransmitters and therefore an increase in monoaminergic neurotransmission.

Levofenfluramine (INN), or (−)-3-trifluoromethyl-N-ethylamphetamine, also known as (−)-fenfluramine or (R)-fenfluramine, is a drug of the amphetamine family that, itself (i.e., in enantiopure form), was never marketed. It is the levorotatory enantiomer of fenfluramine, the racemic form of the compound, whereas the dextrorotatory enantiomer is dexfenfluramine. Both fenfluramine and dexfenfluramine are anorectic agents that have been used clinically in the treatment of obesity (and hence, levofenfluramine has been as well since it is a component of fenfluramine). However, they have since been discontinued due to reports of causing cardiovascular conditions such as valvular heart disease and pulmonary hypertension, adverse effects that are likely to be caused by excessive stimulation of 5-HT2B receptors expressed on heart valves.

Pseudophenmetrazine is a psychostimulant compound of the morpholine class. It is the N-demethylated and cis-configured analogue of phendimetrazine as well as the cis-configured stereoisomer of phenmetrazine. In addition, along with phenmetrazine, it is believed to be one of the active metabolites of phendimetrazine, which itself is inactive and behaves merely as a prodrug. Relative to phenmetrazine, pseudophenmetrazine is of fairly low potency, acting as a modest releasing agent of norepinephrine (EC50 = 514 nM), while its (+)-enantiomer is a weak releaser of dopamine (EC50 = 1,457 nM) whereas its (−)-enantiomer is a weak reuptake inhibitor of dopamine (Ki = 2,691 nM); together as a racemic mixture with the two enantiomers combined, pseudophenmetrazine behaves overall more as a dopamine reuptake inhibitor (Ki = 2,630 nM), possibly due to the (+)-enantiomer blocking the uptake of the (−)-enantiomer into dopaminergic neurons and thus preventing it from inducing dopamine release. Neither enantiomer has any significant effect on serotonin reuptake or release (both Ki = >10,000 nM and EC50 = >10,000 nM, respectively).

Hydroxybupropion, or 6-hydroxybupropion, is the major active metabolite of the antidepressant and smoking cessation drug bupropion. It is formed from bupropion by the liver enzyme CYP2B6 during first-pass metabolism. With oral bupropion treatment, hydroxybupropion is present in plasma at area under the curve concentrations that are as many as 16–20 times greater than those of bupropion itself, demonstrating extensive conversion of bupropion into hydroxybupropion in humans. As such, hydroxybupropion is likely to play a very important role in the effects of oral bupropion, which could accurately be thought of as functioning largely as a prodrug to hydroxybupropion. Other metabolites of bupropion besides hydroxybupropion include threohydrobupropion and erythrohydrobupropion.

Desmethylsibutramine is an active metabolite of the anorectic drug sibutramine. It is a more potent monoamine reuptake inhibitor than sibutramine and has been sold as an ingredient in weight loss products sold as dietary supplements, along with related compounds such as the N-ethyl and 3,4-dichloro derivatives.

Threohydrobupropion is a substituted amphetamine derivative—specifically a β-hydroxyamphetamine—and a major active metabolite of the antidepressant drug bupropion (Wellbutrin). Bupropion is a norepinephrine–dopamine reuptake inhibitor and nicotinic acetylcholine receptor negative allosteric modulator, with its metabolites contributing substantially to its activities. Threohydrobupropion exists as two isomers, (1R,2R)-threohydrobupropion and (1S,2S)-threohydrobupropion. Other metabolites of bupropion include hydroxybupropion and erythrohydrobupropion.

Chlorosipentramine is an analogue of the anorectic drug sibutramine, which has been sold as an ingredient in weight loss products sold as dietary supplements, first detected in South Korea in 2017. It is one of a number of sibutramine derivatives which have been sold in grey-market weight loss products since sibutramine itself was taken off the market due to safety concerns. Others include desmethylsibutramine, didesmethylsibutramine, homosibutramine, chlorosibutramine, and benzylsibutramine. Chlorosipentramine is illegal in South Korea along with other related compounds.
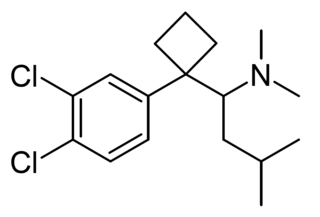
Chlorosibutramine is an analogue of the anorectic drug sibutramine, which has been sold as an ingredient in weight loss products sold as dietary supplements, first detected in South Korea in 2013. It is illegal in South Korea.















