
Non-steroidal anti-inflammatory drugs (NSAID) are members of a therapeutic drug class which reduces pain, decreases inflammation, decreases fever, and prevents blood clots. Side effects depend on the specific drug, its dose and duration of use, but largely include an increased risk of gastrointestinal ulcers and bleeds, heart attack, and kidney disease.

Tramadol, sold under the brand name Ultram among others, is an opioid pain medication and a serotonin–norepinephrine reuptake inhibitor (SNRI) used to treat moderately severe pain. When taken by mouth in an immediate-release formulation, the onset of pain relief usually begins within an hour. It is also available by injection. It is available in combination with paracetamol (acetaminophen).
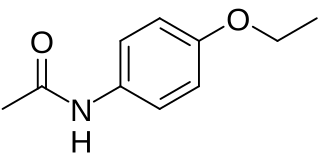
Phenacetin is a pain-relieving and fever-reducing drug, which was widely used following its introduction in 1887. It was withdrawn from medicinal use as dangerous from the 1970s.

An imidazopyridine is a nitrogen containing heterocycle that is also a class of drugs that contain this same chemical substructure. In general, they are GABAA receptor agonists, however recently proton pump inhibitors, aromatase inhibitors, NSAIDs and other classes of drugs in this class have been developed as well. Despite usually being similar to them in effect, they are not chemically related to benzodiazepines. As such, GABAA-agonizing imidazopyridines, pyrazolopyrimidines, and cyclopyrrones are sometimes grouped together and referred to as "nonbenzodiazepines." Imidazopyridines include:

Dextropropoxyphene is an analgesic in the opioid category, patented in 1955 and manufactured by Eli Lilly and Company. It is an optical isomer of levopropoxyphene. It is intended to treat mild pain and also has antitussive and local anaesthetic effects. The drug has been taken off the market in Europe and the US due to concerns of fatal overdoses and heart arrhythmias. It is still available in Australia, albeit with restrictions after an application by its manufacturer to review its proposed banning. Its onset of analgesia is said to be 20–30 minutes and peak effects are seen about 1.5–2.0 hours after oral administration.

Phenazone is an analgesic, antipyretic and anti-inflammatory drug. While it predates the term, it is often classified as a nonsteroidal anti-inflammatory drug (NSAID). Phenazone was one of the earliest synthetic medications — when it was patented in 1883, the only synthetic medical chemicals on the market were chloral hydrate, a sedative, trimethylamine, and iodol (tetraiodopyrrol), an early antiseptic. One of the earliest widely used analgesics and antipyretics, phenazone was gradually replaced in common use by other medications including phenacetin, aspirin, paracetamol and modern NSAIDs such as ibuprofen. However, it is still available in several countries either as an over-the-counter or prescribed drug.

Metamizole or dipyrone is a painkiller, spasm reliever, and fever reliever drug. It is most commonly given by mouth or by intravenous infusion. It belongs to the ampyrone sulfonate family of medicines and was patented in 1922. Metamizole is marketed under various trade names. It was first used medically in Germany under the brandname "Novalgin".

Zomepirac is an orally effective nonsteroidal anti-inflammatory drug (NSAID) that has antipyretic actions. It was developed by McNeil Pharmaceutical, approved by the FDA in 1980, and sold as the sodium salt zomepirac sodium, under the brand name Zomax. Due to its clinical effectiveness, it was preferred by doctors in many situations and obtained a large share of the analgesics market; however, it was subsequently withdrawn in March 1983 due to its tendency to cause serious anaphylaxis in a small, but unpredictable, subset of the patient population.

Desmetramadol, also known as O-desmethyltramadol (O-DSMT), is an opioid analgesic and the main active metabolite of tramadol. Tramadol is demethylated by the liver enzyme CYP2D6 to desmetramadol in the same way as codeine, and so similarly to the variation in effects seen with codeine, individuals who have a less active form of CYP2D6 will tend to have reduced analgesic effects from tramadol. Because desmetramadol itself does not need to be metabolized to induce an analgesic effect, it can be used in individuals with low CYP2D6 activity unlike tramadol.

Flupirtine is an aminopyridine that functions as a centrally acting non-opioid analgesic that was originally used as an analgesic for acute and chronic pain but in 2013 due to issues with liver toxicity, the European Medicines Agency restricted its use to acute pain, for no more than two weeks, and only for people who cannot use other painkillers. In March 2018, marketing authorisations for flupirtine were withdrawn following a European Medicines Agency recommendation based on the finding that the restrictions introduced in 2013 had not been sufficiently followed in clinical practice, and cases of serious liver injury still occurred including liver failure.
Fenclozic acid is an analgesic, antipyretic, and anti-inflammatory drug. It has been withdrawn in 1970 due to jaundice.
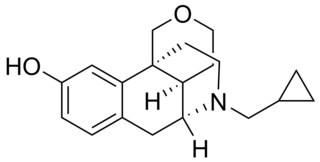
Proxorphan (INN), also known as proxorphan tartate (USAN), is an opioid analgesic and antitussive drug of the morphinan family that was never marketed. It acts preferentially as a κ-opioid receptor partial agonist and to a lesser extent as a μ-opioid receptor partial agonist.

Isomethadone (INN, BAN; trade name Liden; also known as isoamidone) is a synthetic opioid analgesic and antitussive related to methadone that was used formerly as a pharmaceutical drug but is now no longer marketed. Isomethadone was used as both an analgesic and antitussive. It binds to and activates both the μ- and δ-opioid receptors, with the (S)-isomer being the more potent of the two enantiomers. Isomethadone is a Schedule II controlled substance in the United States, with an ACSCN of 9226 and a 2014 aggregate manufacturing quota of 5 g. The salts in use are the hydrobromide (HBr, free base conversion ratio 0.793), hydrochloride (HCl, 0.894), and HCl monohydrate (0.850). Isomethadone is also regulated internationally as a Schedule I controlled substance under the United Nations Single Convention on Narcotic Drugs of 1961.

MT-45 (IC-6) is an opioid analgesic drug invented in the 1970s by Dainippon Pharmaceutical Co. It is chemically a 1-substituted-4-(1,2-diphenylethyl) piperazine derivative, which is structurally unrelated to most other opioid drugs. Racemic MT-45 has around 80% the potency of morphine, with almost all opioid activity residing in the (S) enantiomer. It has been used as a lead compound from which a large family of potent opioid drugs have been developed, including full agonists, partial agonists, and antagonists at the three main opioid receptor subtypes. Fluorinated derivatives of MT-45 such as 2F-MT-45 are significantly more potent as μ-opioid receptor agonists, and one of its main metabolites 1,2-diphenylethylpiperazine also blocks NMDA receptors.

Cinnamedrine, also known as N-cinnamylephedrine, is a sympathomimetic drug with similar effects relative to those of ephedrine. It also has some local anesthetic activity. Cinnamedrine was previously used, in combination with analgesics, as an antispasmodic to treat dysmenorrhea in the over-the-counter drug Midol in the 1980s. There is a case report of the drug being abused as a psychostimulant.
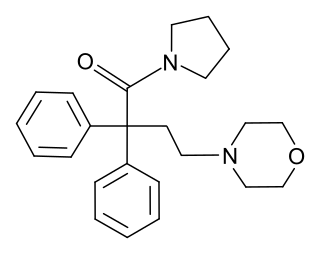
Desmethylmoramide (INN) is an opioid analgesic related to dextromoramide that was synthesized and characterized in the late 1950s but was never marketed.

Difenamizole (INN; brand name Pasalin; former developmental code name AP-14) is a nonsteroidal anti-inflammatory drug (NSAID) and analgesic of the pyrazolone group related to metamizole. It has monoaminergic properties, including inhibition of monoamine oxidase, augmentation of pargyline-induced elevation of striatal dopamine levels, inhibition of K+-induced striatal dopamine release, and inhibition of the reuptake of dopamine.

Anagestone acetate, sold under the brand names Anatropin and Neo-Novum, is a progestin medication which was withdrawn from medical use due to carcinogenicity observed in animal studies.
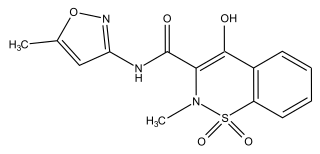
Isoxicam is a nonsteroidal anti-inflammatory drug (NSAID) that was taken or applied to reduce inflammation and as an analgesic reducing pain in certain conditions. The drug was introduced in 1983 by the Warner-Lambert Company. Isoxicam is a chemical analog of piroxicam (Feldene) which has a pyridine ring in lieu of an isoxazole ring. In 1985 isoxicam was withdrawn from the French market, due to adverse effects, namely toxic epidermal necrolysis resulting in death. Although these serious side effects were observed only in France, the drug was withdrawn worldwide.
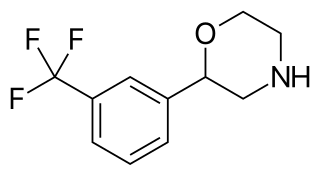
Flumexadol (INN) is a drug described and researched as a non-opioid analgesic which was never marketed. It has been found to act as an agonist of the serotonin 5-HT1A and 5-HT2C receptors and, to a much lesser extent, of the 5-HT2A receptor. According to Nilsson (2006) in a paper on 5-HT2C receptor agonists as potential anorectics, "The (+)-enantiomer of this compound showed [...] affinity for the 5-HT2C receptor (Ki) 25 nM) [...] and was 40-fold selective over the 5-HT2A receptor in receptor binding studies. Curiously, the racemic version [...], also known as 1841 CERM, was originally reported to possess analgesic properties while no association with 5-HT2C receptor activity was mentioned." It is implied that flumexadol might be employable as an anorectic in addition to analgesic. Though flumexadol itself has never been approved for medical use, oxaflozane is a prodrug of the compound that was formerly used clinically in France as an antidepressant and anxiolytic agent.



















