
The prostaglandins (PG) are a group of physiologically active lipid compounds called eicosanoids having diverse hormone-like effects in animals. Prostaglandins have been found in almost every tissue in humans and other animals. They are derived enzymatically from the fatty acid arachidonic acid. Every prostaglandin contains 20 carbon atoms, including a 5-carbon ring. They are a subclass of eicosanoids and of the prostanoid class of fatty acid derivatives.
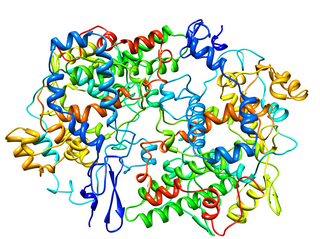
Cyclooxygenase (COX), officially known as prostaglandin-endoperoxide synthase (PTGS), is an enzyme that is responsible for formation of prostanoids, including thromboxane and prostaglandins such as prostacyclin, from arachidonic acid. A member of the animal-type heme peroxidase family, it is also known as prostaglandin G/H synthase. The specific reaction catalyzed is the conversion from arachidonic acid to prostaglandin H2 via a short-living prostaglandin G2 intermediate.
gamma-Linolenic acid or GLA is a fatty acid found primarily in seed oils. When acting on GLA, arachidonate 5-lipoxygenase produces no leukotrienes and the conversion by the enzyme of arachidonic acid to leukotrienes is inhibited.

Prostacyclin (also called prostaglandin I2 or PGI2) is a prostaglandin member of the eicosanoid family of lipid molecules. It inhibits platelet activation and is also an effective vasodilator.
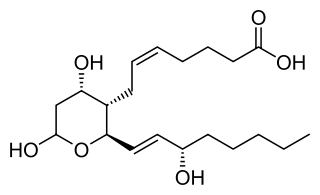
Thromboxane is a member of the family of lipids known as eicosanoids. The two major thromboxanes are thromboxane A2 and thromboxane B2. The distinguishing feature of thromboxanes is a 6-membered ether-containing ring.
Prostanoids are active lipid mediators that regulate inflammatory response. Prostanoids are a subclass of eicosanoids consisting of the prostaglandins, the thromboxanes, and the prostacyclins. Prostanoids are seen to target NSAIDS which allow for therapeutic potential. Prostanoids are present within areas of the body such as the gastrointestinal tract, urinary tract, respiratory and cardiology systems, reproductive tract and vascular system. Prostanoids can even be seen with aid to the water and ion transportation within cells. Prostanoids help release prostaglandins upon activation, receptors may open possibilities for treatments within different systems.

Thromboxane A synthase 1 , also known as TBXAS1, is a cytochrome P450 enzyme that, in humans, is encoded by the TBXAS1 gene.
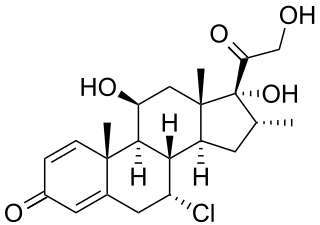
Alclometasone is a synthetic corticosteroid for topical dermatologic use, possessing anti-inflammatory, antipruritic, and vasoconstrictive properties.
Dihomo-γ-linolenic acid (DGLA) is a 20-carbon ω−6 fatty acid. In physiological literature, it is given the name 20:3 (ω−6). DGLA is a carboxylic acid with a 20-carbon chain and three cis double bonds; the first double bond is located at the sixth carbon from the omega end. DGLA is the elongation product of γ-linolenic acid. GLA, in turn, is a desaturation product of linoleic acid. DGLA is made in the body by the elongation of GLA, by an efficient enzyme which does not appear to suffer any form of (dietary) inhibition. DGLA is an extremely uncommon fatty acid, found only in trace amounts in animal products.

The thromboxane receptor (TP) also known as the prostanoid TP receptor is a protein that in humans is encoded by the TBXA2R gene, The thromboxane receptor is one among the five classes of prostanoid receptors and was the first eicosanoid receptor cloned. The TP receptor derives its name from its preferred endogenous ligand thromboxane A2.

Thromboxane B2 is an inactive metabolite/product of thromboxane A2. It is almost completely cleared in the urine.

Thromboxane A2 (TXA2) is a type of thromboxane that is produced by activated platelets during hemostasis and has prothrombotic properties: it stimulates activation of new platelets as well as increases platelet aggregation. This is achieved by activating the thromboxane receptor, which results in platelet-shape change, inside-out activation of integrins, and degranulation. Circulating fibrinogen binds these receptors on adjacent platelets, further strengthening the clot. Thromboxane A2 is also a known vasoconstrictor and is especially important during tissue injury and inflammation. It is also regarded as responsible for Prinzmetal's angina.

Triflusal is a platelet aggregation inhibitor that was discovered and developed in the Uriach Laboratories, and commercialised in Spain since 1981. Currently, it is available in 25 countries in Europe, Asia, Africa and America. It is a derivative of acetylsalicylic acid (ASA) in which a hydrogen atom on the benzene ring has been replaced by a trifluromethyl group. Trade names include Disgren, Grendis, Aflen and Triflux.

Picotamide is a platelet aggregation inhibitor. It works as a thromboxane synthase inhibitor and a thromboxane receptor inhibitor, the latter by modifying cellular responses to activation of the thromboxane receptor. Picotamide is licensed in Italy for the treatment of clinical arterial thrombosis and peripheral artery disease.

Aspirin causes several different effects in the body, mainly the reduction of inflammation, analgesia, the prevention of clotting, and the reduction of fever. Much of this is believed to be due to decreased production of prostaglandins and TXA2. Aspirin's ability to suppress the production of prostaglandins and thromboxanes is due to its irreversible inactivation of the cyclooxygenase (COX) enzyme. Cyclooxygenase is required for prostaglandin and thromboxane synthesis. Aspirin acts as an acetylating agent where an acetyl group is covalently attached to a serine residue in the active site of the COX enzyme. This makes aspirin different from other NSAIDs, which are reversible inhibitors. However, other effects of aspirin, such as uncoupling oxidative phosphorylation in mitochondria, and the modulation of signaling through NF-κB, are also being investigated. Some of its effects are like those of salicylic acid, which is not an acetylating agent.
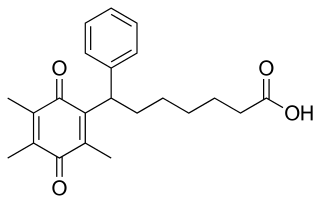
Seratrodast (development name, AA-2414; marketed originally as Bronica) is a thromboxane A2 (TXA2) receptor (TP receptor) antagonist used primarily in the treatment of asthma. It was the first TP receptor antagonist that was developed as an anti-asthmatic drug and received marketing approval in Japan in 1997. As of 2017 seratrodast was marketed as Bronica in Japan, and as Changnuo, Mai Xu Jia, Quan Kang Nuo in China.

Terbogrel (INN) is an experimental drug that has been studied for its potential to prevent the vasoconstricting and platelet-aggregating action of thromboxanes. Terbogrel is an orally available thromboxane A2 receptor antagonist and a thromboxane A synthase inhibitor. The drug was developed by Boehringer Ingelheim.

Dazoxiben is an orally active thromboxane synthase inhibitor. It has shown a significant clinical improvement in patients with Raynaud's syndrome.

20-Hydroxyeicosatetraenoic acid, also known as 20-HETE or 20-hydroxy-5Z,8Z,11Z,14Z-eicosatetraenoic acid, is an eicosanoid metabolite of arachidonic acid that has a wide range of effects on the vascular system including the regulation of vascular tone, blood flow to specific organs, sodium and fluid transport in the kidney, and vascular pathway remodeling. These vascular and kidney effects of 20-HETE have been shown to be responsible for regulating blood pressure and blood flow to specific organs in rodents; genetic and preclinical studies suggest that 20-HETE may similarly regulate blood pressure and contribute to the development of stroke and heart attacks. Additionally the loss of its production appears to be one cause of the human neurological disease, Hereditary spastic paraplegia. Preclinical studies also suggest that the overproduction of 20-HETE may contribute to the progression of certain human cancers, particularly those of the breast.

Furegrelate, also known as 5-(3-pyridinylmethyl)benzofurancarboxylic acid, is a chemical compound with thromboxane enzyme inhibiting properties that was originally developed by Pharmacia Corporation as a drug to treat arrhythmias, ischaemic heart disorders, and thrombosis but was discontinued. It is commercially available in the form furegrelate sodium salt.
















