In pharmacology and toxicology, a route of administration is the way by which a drug, fluid, poison, or other substance is taken into the body.

Pulmonary hypertension is a condition of increased blood pressure in the arteries of the lungs. Symptoms include shortness of breath, fainting, tiredness, chest pain, swelling of the legs, and a fast heartbeat. The condition may make it difficult to exercise. Onset is typically gradual. According to the definition at the 6th World Symposium of Pulmonary Hypertension in 2018, a patient is deemed to have pulmonary hypertension if the pulmonary mean arterial pressure is greater than 20mmHg at rest, revised down from a purely arbitrary 25mmHg, and pulmonary vascular resistance (PVR) greater than 3 Wood units.
Atenolol is a beta blocker medication primarily used to treat high blood pressure and heart-associated chest pain. Atenolol, however, does not seem to improve mortality in those with high blood pressure. Other uses include the prevention of migraines and treatment of certain irregular heart beats. It is taken orally or by intravenous injection. It can also be used with other blood pressure medications.

Prostacyclin (also called prostaglandin I2 or PGI2) is a prostaglandin member of the eicosanoid family of lipid molecules. It inhibits platelet activation and is also an effective vasodilator.

Loop diuretics are diuretics that act on the Na-K-Cl cotransporter along the thick ascending limb of the loop of Henle in nephrons of the kidneys. They are primarily used in medicine to treat hypertension and edema often due to congestive heart failure or chronic kidney disease. While thiazide diuretics are more effective in patients with normal kidney function, loop diuretics are more effective in patients with impaired kidney function.
Remifentanil, marketed under the brand name Ultiva is a potent, short-acting synthetic opioid analgesic drug. It is given to patients during surgery to relieve pain and as an adjunct to an anaesthetic. Remifentanil is used for sedation as well as combined with other medications for use in general anesthesia. The use of remifentanil has made possible the use of high-dose opioid and low-dose hypnotic anesthesia, due to synergism between remifentanil and various hypnotic drugs and volatile anesthetics.

Nimodipine, sold under the brand name Nimotop among others, is calcium channel blocker used in preventing vasospasm secondary to subarachnoid hemorrhage. It was originally developed within the calcium channel blocker class as it was used for the treatment of high blood pressure, but is not used for this indication.
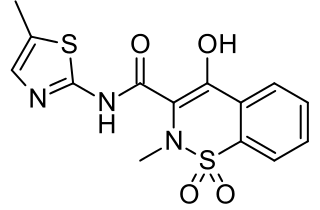
Meloxicam, sold under the brand name Mobic among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain and inflammation in rheumatic diseases and osteoarthritis. It is used by mouth or by injection into a vein. It is recommended that it be used for as short a period as possible and at a low dose.

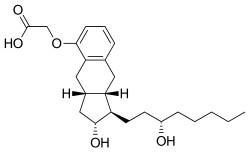
Iloprost, sold under the brand name Ventavis among others, is a medication used to treat pulmonary arterial hypertension (PAH), scleroderma, Raynaud's phenomenon, frostbite, and other conditions in which the blood vessels are constricted and blood cannot flow to the tissues. Iloprost is a prostacyclin mimetic.

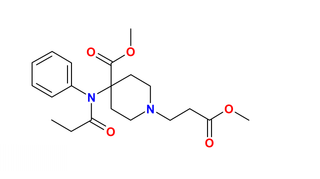
Fenoldopam mesylate (Corlopam) is a drug and synthetic benzazepine derivative which acts as a selective D1 receptor partial agonist. Fenoldopam is used as an antihypertensive agent. It was approved by the Food and Drug Administration (FDA) in September 1997.
Portopulmonary hypertension (PPH) is defined by the coexistence of portal and pulmonary hypertension. PPH is a serious complication of liver disease, present in 0.25 to 4% of all patients with cirrhosis. Once an absolute contraindication to liver transplantation, it is no longer, thanks to rapid advances in the treatment of this condition. Today, PPH is comorbid in 4-6% of those referred for a liver transplant.

Beraprost is a pharmaceutical drug used in several Asian countries, including Japan and South Korea, as a vasodilator and antiplatelet agent. It is classified as a prostacyclin analog.

The Prostacyclin receptor, also termed the prostaglandin I2 receptor or just IP, is a receptor belonging to the prostaglandin (PG) group of receptors. IP binds to and mediates the biological actions of prostacyclin (also termed Prostaglandin I2, PGI2, or when used as a drug, epoprostenol). IP is encoded in humans by the PTGIR gene. While possessing many functions as defined in animal model studies, the major clinical relevancy of IP is as a powerful vasodilator: stimulators of IP are used to treat severe and even life-threatening diseases involving pathological vasoconstriction.

Clevidipine is a dihydropyridine calcium channel blocker indicated for the reduction of blood pressure when oral therapy is not feasible or not desirable. Clevidipine is used IV only and practitioners titrate this drug to lower blood pressure. It has a half-life of approximately one minute. It is rapidly inactivated by esterases.

Actelion is a pharmaceuticals and biotechnology company established in December 1997, headquartered in Allschwil near Basel, Switzerland.

United Therapeutics Corporation is an American publicly traded biotechnology company and public benefit corporation listed on the NASDAQ under the symbol UTHR. It develops novel, life-extending technologies for patients in the areas of lung disease and organ manufacturing. United Therapeutics is co-headquartered in Silver Spring, Maryland and Research Triangle Park, North Carolina, with additional facilities in Magog and Bromont, Quebec; Melbourne and Jacksonville, Florida; Blacksburg, Virginia; and Manchester, New Hampshire.
Recombinant factor VIIa (rfVIIa) is a form of blood factor VII that has been manufactured via recombinant technology. It is administered via an injection into a vein. It is used to treat bleeding episodes in people who have acquired haemophilia, among other indications. There are several disimilar forms, and biosimilars for each. All forms are activated.

Selexipag, sold under the brand name Uptravi, is a medication developed by Actelion for the treatment of pulmonary arterial hypertension (PAH). Selexipag and its active metabolite, ACT-333679, are agonists of the prostacyclin receptor, which leads to vasodilation in the pulmonary circulation. It is taken by mouth or administered intravenously.

Angiotensin II is a medication that is used to treat hypotension resulting from septic shock or other distributive shock. It is a synthetic vasoconstrictor peptide that is identical to human hormone angiotensin II and is marketed under the brand name Giapreza. The Food and Drug Administration approved the use of angiotensin II in December 2017 to treat low blood pressure resulting from septic shock.

Furegrelate, also known as 5-(3-pyridinylmethyl)benzofurancarboxylic acid, is a chemical compound with thromboxane enzyme inhibiting properties that was originally developed by Pharmacia Corporation as a drug to treat arrhythmias, ischaemic heart disorders, and thrombosis but was discontinued. It is commercially available in the form furegrelate sodium salt.