Aspirin, also known as acetylsalicylic acid (ASA), is a nonsteroidal anti-inflammatory drug (NSAID) used to reduce pain, fever, and/or inflammation, and as an antithrombotic. Specific inflammatory conditions which aspirin is used to treat include Kawasaki disease, pericarditis, and rheumatic fever.
An antiplatelet drug (antiaggregant), also known as a platelet agglutination inhibitor or platelet aggregation inhibitor, is a member of a class of pharmaceuticals that decrease platelet aggregation and inhibit thrombus formation. They are effective in the arterial circulation where classical Vitamin K antagonist anticoagulants have minimal effect.

An anticoagulant, commonly known as a blood thinner, is a chemical substance that prevents or reduces the coagulation of blood, prolonging the clotting time. Some occur naturally in blood-eating animals, such as leeches and mosquitoes, which help keep the bite area unclotted long enough for the animal to obtain blood.
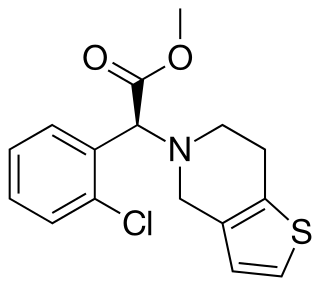
Clopidogrel, sold under the brand name Plavix among others, is an antiplatelet medication used to reduce the risk of heart disease and stroke in those at high risk. It is also used together with aspirin in heart attacks and following the placement of a coronary artery stent. It is taken by mouth. Its effect starts about two hours after intake and lasts for five days.

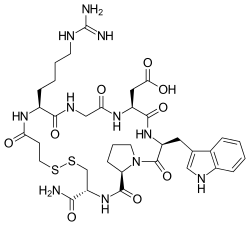
Abciximab, a glycoprotein IIb/IIIa receptor antagonist manufactured by Janssen Biologics BV and distributed by Eli Lilly under the trade name ReoPro, is a platelet aggregation inhibitor mainly used during and after coronary artery procedures like angioplasty to prevent platelets from sticking together and causing thrombus formation within the coronary artery. It is a glycoprotein IIb/IIIa inhibitor.

Glanzmann's thrombasthenia is an abnormality of the platelets. It is an extremely rare coagulopathy, in which the platelets contain defective or low levels of glycoprotein IIb/IIIa (GpIIb/IIIa), which is a receptor for fibrinogen. As a result, no fibrinogen bridging of platelets to other platelets can occur, and the bleeding time is significantly prolonged.

Thromboxane is a member of the family of lipids known as eicosanoids. The two major thromboxanes are thromboxane A2 and thromboxane B2. The distinguishing feature of thromboxanes is a 6-membered ether-containing ring.
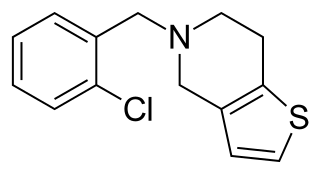
Ticlopidine, sold under the brand name Ticlid, is a medication used to reduce the risk of thrombotic strokes. It is an antiplatelet drug in the thienopyridine family which is an adenosine diphosphate (ADP) receptor inhibitor. Research initially showed that it was useful for preventing strokes and coronary stent occlusions. However, because of its rare but serious side effects of neutropenia and thrombotic microangiopathy it was primarily used in patients in whom aspirin was not tolerated, or in whom dual antiplatelet therapy was desirable. With the advent of newer and safer antiplatelet drugs such as clopidogrel and ticagrelor, its use remained limited.

Dipyridamole is a nucleoside transport inhibitor and a PDE3 inhibitor medication that inhibits blood clot formation when given chronically and causes blood vessel dilation when given at high doses over a short time.
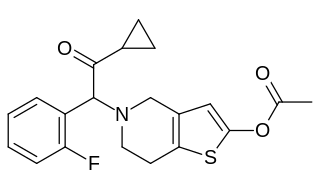
Prasugrel, sold under the brand name Effient in the US, Australia and India, and Efient in the EU) is a medication used to prevent formation of blood clots. It is a platelet inhibitor and an irreversible antagonist of P2Y12 ADP receptors and is of the thienopyridine drug class. It was developed by Daiichi Sankyo Co. and produced by Ube and marketed in the United States in cooperation with Eli Lilly and Company.
In biochemistry and medicine, glycoprotein IIb/IIIa is an integrin complex found on platelets. It is a transmembrane receptor for fibrinogen and von Willebrand factor, and aids platelet activation. The complex is formed via calcium-dependent association of gpIIb and gpIIIa, a required step in normal platelet aggregation and endothelial adherence. Platelet activation by ADP leads to the aforementioned conformational change in platelet gpIIb/IIIa receptors that induces binding to fibrinogen. The gpIIb/IIIa receptor is a target of several drugs including abciximab, eptifibatide, and tirofiban.

Tirofiban, sold under the brand name Aggrastat, is an antiplatelet medication. It belongs to a class of antiplatelets named glycoprotein IIb/IIIa inhibitors. Tirofiban is a small molecule inhibitor of the protein-protein interaction between fibrinogen and the platelet integrin receptor GP IIb/IIIa and is the first drug candidate whose origins can be traced to a pharmacophore-based virtual screening lead.

P2Y12 is a chemoreceptor for adenosine diphosphate (ADP) that belongs to the Gi class of a group of G protein-coupled (GPCR) purinergic receptors. This P2Y receptor family has several receptor subtypes with different pharmacological selectivity, which overlaps in some cases, for various adenosine and uridine nucleotides. The P2Y12 receptor is involved in platelet aggregation and is thus a biological target for the treatment of thromboembolisms and other clotting disorders. Two transcript variants encoding the same isoform have been identified for this gene.

Bivalirudin, sold under the brand names Angiomax and Angiox, among others, is a specific and reversible direct thrombin inhibitor (DTI). Chemically, it is a synthetic congener of the naturally occurring drug hirudin, found in the saliva of the medicinal leech Hirudo medicinalis. It is manufactured by The Medicines Company.

Vorapaxar is a thrombin receptor antagonist based on the natural product himbacine, discovered by Schering-Plough and developed by Merck & Co.

Ticagrelor, sold under the brand name Brilinta among others, is a medication used for the prevention of stroke, heart attack and other events in people with acute coronary syndrome, meaning problems with blood supply in the coronary arteries. It acts as a platelet aggregation inhibitor by antagonising the P2Y12 receptor. The drug is produced by AstraZeneca.

Cangrelor, sold under the brand name Kengreal among others, is a P2Y12 inhibitor FDA approved as of June 2015 as an antiplatelet drug for intravenous application. Some P2Y12 inhibitors are used clinically as effective inhibitors of adenosine diphosphate-mediated platelet activation and aggregation. Unlike clopidogrel (Plavix), which is a prodrug, cangrelor is an active drug not requiring metabolic conversion.
Adenosine diphosphate (ADP) receptor inhibitors are a drug class of antiplatelet agents, used in the treatment of acute coronary syndrome (ACS) or in preventive treatment for patients who are in risk of thromboembolism, myocardial infarction or a stroke. These drugs antagonize the P2Y12 platelet receptors and therefore prevent the binding of ADP to the P2Y12 receptor. This leads to a decrease in aggregation of platelets, prohibiting thrombus formation. The P2Y12 receptor is a surface bound protein found on blood platelets. They belong to G protein-coupled purinergic receptors (GPCR) and are chemoreceptors for ADP.

Reperfusion therapy is a medical treatment to restore blood flow, either through or around, blocked arteries, typically after a heart attack. Reperfusion therapy includes drugs and surgery. The drugs are thrombolytics and fibrinolytics used in a process called thrombolysis. Surgeries performed may be minimally-invasive endovascular procedures such as a percutaneous coronary intervention (PCI), which involves coronary angioplasty. The angioplasty uses the insertion of a balloon and/or stents to open up the artery. Other surgeries performed are the more invasive bypass surgeries that graft arteries around blockages.

Management of acute coronary syndrome is targeted against the effects of reduced blood flow to the affected area of the heart muscle, usually because of a blood clot in one of the coronary arteries, the vessels that supply oxygenated blood to the myocardium. This is achieved with urgent hospitalization and medical therapy, including drugs that relieve chest pain and reduce the size of the infarct, and drugs that inhibit clot formation; for a subset of patients invasive measures are also employed. Basic principles of management are the same for all types of acute coronary syndrome. However, some important aspects of treatment depend on the presence or absence of elevation of the ST segment on the electrocardiogram, which classifies cases upon presentation to either ST segment elevation myocardial infarction (STEMI) or non-ST elevation acute coronary syndrome (NST-ACS); the latter includes unstable angina and non-ST elevation myocardial infarction (NSTEMI). Treatment is generally more aggressive for STEMI patients, and reperfusion therapy is more often reserved for them. Long-term therapy is necessary for prevention of recurrent events and complications.