Aspirin, also known as acetylsalicylic acid (ASA), is a nonsteroidal anti-inflammatory drug (NSAID) used to reduce pain, fever, and/or inflammation, and as an antithrombotic. Specific inflammatory conditions which aspirin is used to treat include Kawasaki disease, pericarditis, and rheumatic fever.
A transient ischemic attack (TIA), commonly known as a mini-stroke, is a minor stroke whose noticeable symptoms usually end in less than an hour. TIA causes the same symptoms associated with strokes, such as weakness or numbness on one side of the body, sudden dimming or loss of vision, difficulty speaking or understanding language, slurred speech, or confusion.
An antiplatelet drug (antiaggregant), also known as a platelet agglutination inhibitor or platelet aggregation inhibitor, is a member of a class of pharmaceuticals that decrease platelet aggregation and inhibit thrombus formation. They are effective in the arterial circulation where anticoagulants have little effect.

Cerebrovascular disease includes a variety of medical conditions that affect the blood vessels of the brain and the cerebral circulation. Arteries supplying oxygen and nutrients to the brain are often damaged or deformed in these disorders. The most common presentation of cerebrovascular disease is an ischemic stroke or mini-stroke and sometimes a hemorrhagic stroke. Hypertension is the most important contributing risk factor for stroke and cerebrovascular diseases as it can change the structure of blood vessels and result in atherosclerosis. Atherosclerosis narrows blood vessels in the brain, resulting in decreased cerebral perfusion. Other risk factors that contribute to stroke include smoking and diabetes. Narrowed cerebral arteries can lead to ischemic stroke, but continually elevated blood pressure can also cause tearing of vessels, leading to a hemorrhagic stroke.
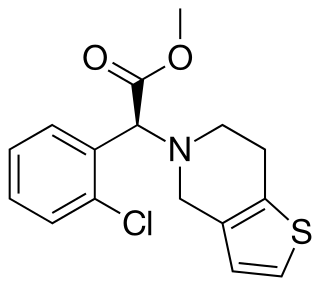
Clopidogrel — sold under the brand name Plavix, among others — is an antiplatelet medication used to reduce the risk of heart disease and stroke in those at high risk. It is also used together with aspirin in heart attacks and following the placement of a coronary artery stent. It is taken by mouth. Its effect starts about two hours after intake and lasts for five days.
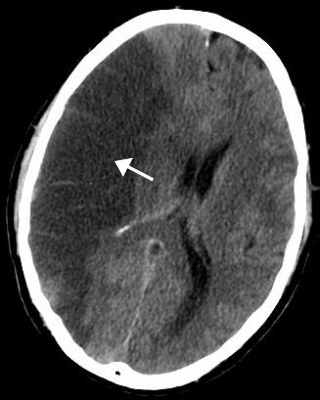
A stroke is a medical condition in which poor blood flow to the brain causes cell death. There are two main types of stroke: ischemic, due to lack of blood flow, and hemorrhagic, due to bleeding. Both cause parts of the brain to stop functioning properly.
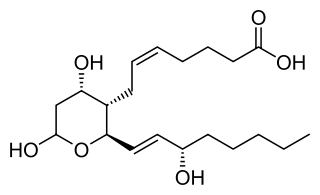
Thromboxane is a member of the family of lipids known as eicosanoids. The two major thromboxanes are thromboxane A2 and thromboxane B2. The distinguishing feature of thromboxanes is a 6-membered ether-containing ring.
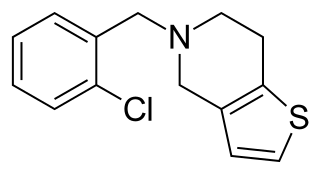
Ticlopidine, sold under the brand name Ticlid, is a medication used to reduce the risk of thrombotic strokes. It is an antiplatelet drug in the thienopyridine family which is an adenosine diphosphate (ADP) receptor inhibitor. Research initially showed that it was useful for preventing strokes and coronary stent occlusions. However, because of its rare but serious side effects of neutropenia and thrombotic thrombocytopenic purpura it was primarily used in patients in whom aspirin was not tolerated, or in whom dual antiplatelet therapy was desirable. With the advent of newer and safer antiplatelet drugs such as clopidogrel and ticagrelor, its use remained limited.

A watershed stroke is defined as a brain ischemia that is localized to the vulnerable border zones between the tissues supplied by the anterior, posterior and middle cerebral arteries. The actual blood stream blockage/restriction site can be located far away from the infarcts. Watershed locations are those border-zone regions in the brain supplied by the major cerebral arteries where blood supply is decreased. Watershed strokes are a concern because they comprise approximately 10% of all ischemic stroke cases. The watershed zones themselves are particularly susceptible to infarction from global ischemia as the distal nature of the vasculature predisposes these areas to be most sensitive to profound hypoperfusion.
The Thrombolysis In Myocardial Infarction, or TIMI Study Group, is an Academic Research Organization (ARO) affiliated with Brigham and Women's Hospital and Harvard Medical School dedicated to advancing the knowledge and care of patients with cardiovascular disease. The TIMI Study Group provides robust expertise in the key aspects of a clinical trial, including academic leadership, global trial management, biostatistics, clinical event adjudication, safety desk, medical hotline, and core laboratories. The group has its headquarters in Boston, Massachusetts.
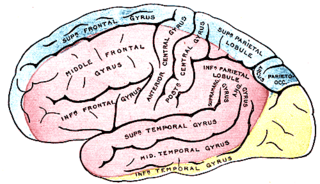
Anterior cerebral artery syndrome is a condition whereby the blood supply from the anterior cerebral artery (ACA) is restricted, leading to a reduction of the function of the portions of the brain supplied by that vessel: the medial aspects of the frontal and parietal lobes, basal ganglia, anterior fornix and anterior corpus callosum.

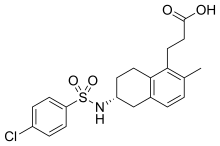
Triflusal is a platelet aggregation inhibitor that was discovered and developed in the Uriach Laboratories, and commercialised in Spain since 1981. Currently, it is available in 25 countries in Europe, Asia, Africa and America. It is a derivative of acetylsalicylic acid (ASA) in which a hydrogen atom on the benzene ring has been replaced by a trifluromethyl group. Trade names include Disgren, Grendis, Aflen and Triflux.

Aspirin causes several different effects in the body, mainly the reduction of inflammation, analgesia, the prevention of clotting, and the reduction of fever. Much of this is believed to be due to decreased production of prostaglandins and TXA2. Aspirin's ability to suppress the production of prostaglandins and thromboxanes is due to its irreversible inactivation of the cyclooxygenase (COX) enzyme. Cyclooxygenase is required for prostaglandin and thromboxane synthesis. Aspirin acts as an acetylating agent where an acetyl group is covalently attached to a serine residue in the active site of the COX enzyme. This makes aspirin different from other NSAIDs, which are reversible inhibitors. However, other effects of aspirin, such as uncoupling oxidative phosphorylation in mitochondria, and the modulation of signaling through NF-κB, are also being investigated. Some of its effects are like those of salicylic acid, which is not an acetylating agent.

Vorapaxar is a thrombin receptor antagonist based on the natural product himbacine, discovered by Schering-Plough and developed by Merck & Co.

Lubeluzole (Prosynap) is a drug which acts as an indirect NMDA antagonist. It inhibits the release of glutamate, inhibits nitric oxide synthesis, and blocks calcium and sodium gated ion channels. It has neuroprotective effects particularly in hypoxic conditions, and was developed for the treatment of stroke. Trials showed it to be safe, effective and well tolerated at low doses, but unfortunately higher doses produced the dangerous cardiac side effect of lengthening the QTc interval, which could potentially lead to heart failure, and so this meant that subsequent trials were limited to using only the low dose range. Animal studies had shown lubeluzole to produce neuroprotective effects when administered for prolonged periods, but the aim of its developers was to produce a drug that would be effective for preventing damage from acute stroke, and so ultimately it failed to show sufficient efficacy in trials and development for medical use was halted.

Ticagrelor, sold under the brand name Brilinta among others, is a medication used for the prevention of stroke, heart attack and other events in people with acute coronary syndrome, meaning problems with blood supply in the coronary arteries. It acts as a platelet aggregation inhibitor by antagonising the P2Y12 receptor. The drug is produced by AstraZeneca.
Adenosine diphosphate (ADP) receptor inhibitors are a drug class of antiplatelet agents, used in the treatment of acute coronary syndrome (ACS) or in preventive treatment for patients who are in risk of thromboembolism, myocardial infarction or a stroke. These drugs antagonize the P2Y12 platelet receptors and therefore prevent the binding of ADP to the P2Y12 receptor. This leads to a decrease in aggregation of platelets, prohibiting thrombus formation. The P2Y12 receptor is a surface bound protein found on blood platelets. They belong to G protein-coupled purinergic receptors (GPCR) and are chemoreceptors for ADP.

Ifetroban is a potent and selective thromboxane receptor antagonist. It has been studied in animal models for the treatment of cancer metastasis, myocardial ischemia, hypertension, stroke, thrombosis, cardiomyopathy, and for its effects on platelets. Clinical trials are evaluating the therapeutic safety and efficacy of oral ifetroban capsules for the treatment of cancer metastasis, cardiovascular disease, aspirin exacerbated respiratory disease, systemic sclerosis, and Duchenne muscular dystrophy.
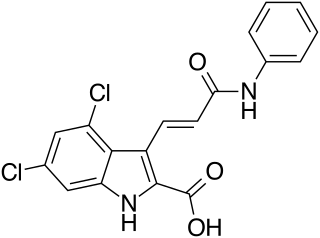
Gavestinel (GV-150,526) was an investigational drug developed by GlaxoSmithKline for acute intracerebral hemorrhage, which in 2001 failed to show an effect in what was at the time, the largest clinical trial in stroke that had been conducted.
Embolic stroke of undetermined source (ESUS) is a type of ischemic stroke with an unknown origin, defined as a non-lacunar brain infarct without proximal arterial stenosis or cardioembolic sources. As such, it forms a subset of cryptogenic stroke, which is part of the TOAST-classification. The following diagnostic criteria define an ESUS: