
Pulmonary hypertension is a condition of increased blood pressure in the arteries of the lungs. Symptoms include shortness of breath, fainting, tiredness, chest pain, swelling of the legs, and a fast heartbeat. The condition may make it difficult to exercise. Onset is typically gradual. According to the definition at the 6th World Symposium of Pulmonary Hypertension in 2018, a patient is deemed to have pulmonary hypertension if the pulmonary mean arterial pressure is greater than 20mmHg at rest, revised down from a purely arbitrary 25mmHg, and pulmonary vascular resistance (PVR) greater than 3 Wood units.

A phosphodiesterase type 5 inhibitor is a vasodilating drug that works by blocking the degradative action of cGMP-specific phosphodiesterase type 5 (PDE5) on cyclic GMP in the smooth muscle cells lining the blood vessels supplying various tissues. These drugs dilate the corpora cavernosa of the penis, facilitating erection with sexual stimulation, and are used in the treatment of erectile dysfunction (ED). Sildenafil was the first effective oral treatment available for ED. Because PDE5 is also present in the smooth muscle of the walls of the arterioles within the lungs, two PDE5 inhibitors, sildenafil and tadalafil, are FDA-approved for the treatment of pulmonary hypertension. As of 2019, the wider cardiovascular benefits of PDE5 inhibitors are being appreciated.
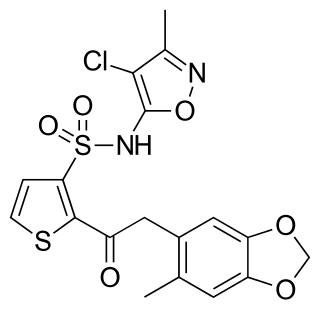
Sitaxentan sodium (TBC-11251) is a medication for the treatment of pulmonary arterial hypertension (PAH). It was marketed as Thelin by Encysive Pharmaceuticals until Pfizer purchased Encysive in February 2008. In 2010, Pfizer voluntarily removed sitaxentan from the market due to concerns about liver toxicity.
An endothelin receptor antagonist (ERA) is a drug that blocks endothelin receptors.

Endothelins are peptides with receptors and effects in many body organs. Endothelin constricts blood vessels and raises blood pressure. The endothelins are normally kept in balance by other mechanisms, but when overexpressed, they contribute to high blood pressure (hypertension), heart disease, and potentially other diseases.

Bosentan, sold under the brand name Tracleer among others, is a dual endothelin receptor antagonist medication used in the treatment of pulmonary artery hypertension (PAH).

Iloprost, sold under the brand name Ventavis among others, is a medication used to treat pulmonary arterial hypertension (PAH), scleroderma, Raynaud's phenomenon, frostbite, and other conditions in which the blood vessels are constricted and blood cannot flow to the tissues. Iloprost is a prostacyclin mimetic.
Portopulmonary hypertension (PPH) is defined by the coexistence of portal and pulmonary hypertension. PPH is a serious complication of liver disease, present in 0.25 to 4% of all patients with cirrhosis. Once an absolute contraindication to liver transplantation, it is no longer, thanks to rapid advances in the treatment of this condition. Today, PPH is comorbid in 4-6% of those referred for a liver transplant.
There are at least four known endothelin receptors, ETA, ETB1, ETB2 and ETC, all of which are G protein-coupled receptors whose activation result in elevation of intracellular-free calcium, which constricts the smooth muscles of the blood vessels, raising blood pressure, or relaxes the smooth muscles of the blood vessels, lowering blood pressure, among other functions.

Ambrisentan, sold under the brand name Letairis among others, is a drug used for the treatment of pulmonary hypertension. It is an endothelin receptor antagonist.

Endothelin 1 (ET-1), also known as preproendothelin-1 (PPET1), is a potent vasoconstrictor peptide produced by vascular endothelial cells. The protein encoded by this gene – EDN1 – is proteolytically processed to release endothelin 1. Endothelin 1 is one of three isoforms of human endothelin.
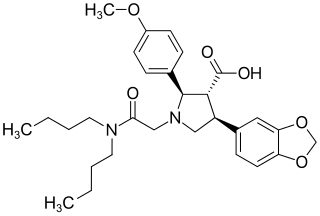
Atrasentan is an experimental drug that is being studied for the treatment of various types of cancer, including non-small cell lung cancer. It is also being investigated as a therapy for diabetic kidney disease.

Endothelin receptor type B, (ET-B) is a protein that in humans is encoded by the EDNRB gene.

Terguride, sold under the brand name Teluron, is a serotonin receptor antagonist and dopamine receptor agonist of the ergoline family. It is approved for and used as a prolactin inhibitor in the treatment of hyperprolactinemia in Japan. Terguride is taken by mouth.

Riociguat, sold under the brand name Adempas, is a medication by Bayer that is a stimulator of soluble guanylate cyclase (sGC). It is used to treat two forms of pulmonary hypertension (PH): chronic thromboembolic pulmonary hypertension (CTEPH) and pulmonary arterial hypertension (PAH). Riociguat constitutes the first drug of the class of sGC stimulators. The drug has a half-life of 12 hours and will decrease dyspnea associated with pulmonary arterial hypertension.

PRX-08066 is a drug discovered and developed by Predix Pharmaceuticals [Dale S. Dhanoa et al. Patent US 7,030,240 B2], which acts as a potent and selective antagonist at the serotonin 5-HT2B receptor, with a 5-HT2Bbinding affinity (Ki) of 3.4nM, and high selectivity over the closely related 5-HT2A and 5-HT2C receptors and other receptor targets. PRX-08066 and other selective 5-HT2B antagonists are being researched for the treatment of pulmonary arterial hypertension, following the discovery that the potent 5-HT2B agonist norfenfluramine produces pulmonary arterial hypertension and subsequent heart valve damage. In animal studies, PRX-08066 has been found to reduce several key indicators of pulmonary arterial hypertension and improved cardiac output, with similar efficacy to established drugs for this condition such as bosentan, sildenafil, beraprost and iloprost. It is also being researched for potential anti-cancer applications, due to its ability to inhibit fibroblast activation.

Actelion is a pharmaceuticals and biotechnology company established in December 1997, headquartered in Allschwil near Basel, Switzerland.

Selexipag, sold under the brand name Uptravi, is a medication developed by Actelion for the treatment of pulmonary arterial hypertension (PAH). Selexipag and its active metabolite, ACT-333679, are agonists of the prostacyclin receptor, which leads to vasodilation in the pulmonary circulation. It is taken by mouth or administered intravenously.

Sarafotoxins (SRTXs) are a group of toxins present in the venom of Atractaspis engaddensis, and in clinical trials cause similar symptoms to patients diagnosed with acute giardiasis. Their etymology is from the name of the snake "שרף עין גדי" in Hebrew, pronounced "Saraf Ein Gedi". Together with endothelins (ETs), they form a homogenous family of strong vasoconstrictor isopeptides. Among them, a few slightly different substances can be named as SRTX-a, SRTX-b, SRTX-c, which were initially derived from A. engaddensis. Each one contains twenty-one amino acid residues that spontaneously fold into a defined tertiary structure, with two interchain-cysteine linkages and a long hydrophobic tail. There are also other compounds, however, they are mostly derivations of previously mentioned ones. The main differences in the family of endothelin and sarafotoxins appear at N-terminal of peptides, as C-terminal in all of them is almost the same.

Aprocitentan, sold under the brand name Tryvio, is a medication used for the treatment of hypertension. It is an endothelin receptor antagonist active at both endothelin A and endothelin B receptors. It is developed for resistant hypertension by Idorsia, which sold it to Janssen but purchased the rights back in 2023 for $343 million.


















