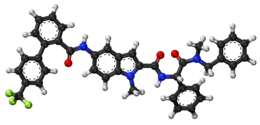
Itraconazole, sometimes abbreviated ITZ, is an antifungal medication used to treat a number of fungal infections. This includes aspergillosis, blastomycosis, coccidioidomycosis, histoplasmosis, and paracoccidioidomycosis. It may be given by mouth or intravenously.
An anorectic or anorexic is a drug which reduces appetite, resulting in lower food consumption, leading to weight loss. By contrast, an appetite stimulant is referred to as orexigenic.
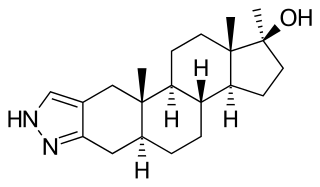
Stanozolol, sold under many brand names, is an androgen and anabolic steroid (AAS) medication derived from dihydrotestosterone (DHT). It is used to treat hereditary angioedema. It was developed by American pharmaceutical company Winthrop Laboratories in 1962, and has been approved by the U.S. Food and Drug Administration for human use, though it is no longer marketed in the USA. It is also used in veterinary medicine. Stanozolol has mostly been discontinued, and remains available in only a few countries. It is given by mouth in humans or by injection into muscle in animals.

Phentermine (phenyl-tertiary-butylamine), with several brand names including Ionamin and Sentis, is a medication used together with diet and exercise to treat obesity. It is taken by mouth for up to a few weeks at a time, after which the benefits subside. It is also available as the combination phentermine/topiramate.
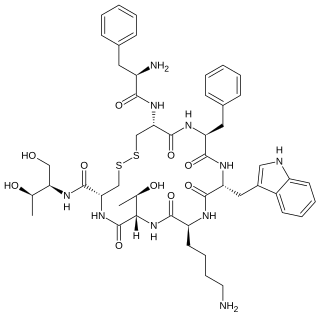
Octreotide, sold under the brand name Sandostatin among others, is an octapeptide that mimics natural somatostatin pharmacologically, though it is a more potent inhibitor of growth hormone, glucagon, and insulin than the natural hormone. It was first synthesized in 1979 by the chemist Wilfried Bauer, and binds predominantly to the somatostatin receptors SSTR2 and SSTR5.

Selamectin is a topical parasiticide and anthelminthic used on dogs and cats. It treats and prevents infections of heartworms, fleas, ear mites, sarcoptic mange (scabies), and certain types of ticks in dogs, and prevents heartworms, fleas, ear mites, hookworms, and roundworms in cats. It is structurally related to ivermectin and milbemycin. Selamectin is not approved for human use.

Carprofen is a nonsteroidal anti-inflammatory drug (NSAID) of the carbazole and propionic acid class that was previously for use in humans and animals but is now only available to veterinarians for prescribing as a supportive treatment for various conditions in animals. Carprofen reduces inflammation by inhibition of COX-1 and COX-2; its specificity for COX-2 varies from species to species. Marketed under many brand names worldwide, carprofen is used as a treatment for inflammation and pain, including joint pain and postoperative pain.

Sitagliptin, sold under the brand name Januvia among others, is an anti-diabetic medication used to treat type 2 diabetes. In the United Kingdom it is listed as less preferred than metformin or a sulfonylurea. It is taken by mouth. It is also available in the fixed-dose combination medication sitagliptin/metformin.

Lubiprostone, sold under the brand name Amitiza among others, is a medication used in the management of chronic idiopathic constipation, predominantly irritable bowel syndrome-associated constipation in women and opioid-induced constipation. The drug is owned by Mallinckrodt and is marketed by Takeda Pharmaceutical Company.
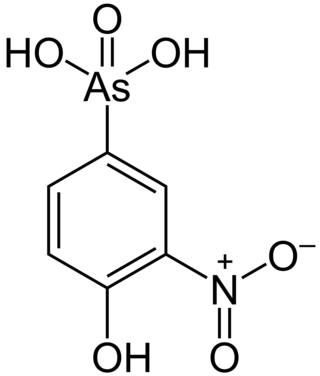
Roxarsone is an organoarsenic compound that has been used in poultry production as a feed additive to increase weight gain and improve feed efficiency, and as a coccidiostat. As of June 2011, it was approved for chicken feed in the United States, Canada, Australia, and 12 other countries. The drug was also approved in the United States and elsewhere for use in pigs.
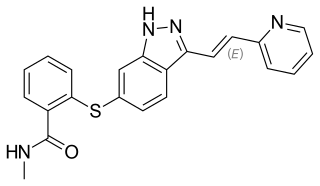
Axitinib, sold under the brand name Inlyta, is a small molecule tyrosine kinase inhibitor developed by Pfizer. It has been shown to significantly inhibit growth of breast cancer in animal (xenograft) models and has shown partial responses in clinical trials with renal cell carcinoma (RCC) and several other tumour types.

Maropitant (INN; brand name: Cerenia, used as maropitant citrate (USAN), is a neurokinin-1 (NK1) receptor antagonist developed by Zoetis specifically for the treatment of motion sickness and vomiting in dogs. It was approved by the FDA in 2007, for use in dogs and in 2012, for cats.

Cefovecin (INN) is an antibiotic of the cephalosporin class, licensed for the treatment of skin infections in cats and dogs. It is marketed by Zoetis under the trade name Convenia. It is used to treat skin infections caused by Pasteurella multocida in cats, and Staphylococcus intermedius and Streptococcus canis in dogs. The advantage of using a long-acting injectable antibiotic is that, unlike with daily administration, doses cannot be missed, which may allow partially resistant microbes to recover. The disadvantage is the presence of subtherapeutic concentrations in the weeks after the resolution of infections. This is associated with the development of resistance in microbes. It should not be used in pregnant or lactating animals or in animals with a history of allergies to penicillin or cephalosporin drugs.

Carindacillin (INN), also known as carbenicillin indanyl (USAN), is a penicillin antibiotic. It is a prodrug of carbenicillin.

Obesity in pets occurs when excessive adipose tissue accumulates in the body, and is generally defined as occurring when an animal's body weight is at least 20% greater than its optimal body weight. Obesity is associated with metabolic and hormonal changes, and can predispose pets to illnesses like orthopedic disease, diabetes, and cancer.

Tofacitinib, sold under the brand Xeljanz among others, is a medication used to treat rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, polyarticular course juvenile idiopathic arthritis, and ulcerative colitis. It is a janus kinase (JAK) inhibitor, discovered and developed by the National Institutes of Health and Pfizer.

Setmelanotide, sold under the brand name Imcivree, is a medication used for the treatment of genetic obesity caused by a rare single-gene mutation.
Semaglutide is an antidiabetic medication used for the treatment of type 2 diabetes and an anti-obesity medication used for long-term weight management. It was developed by Novo Nordisk It was approved for use in the US in 2017. It is a peptide similar to the hormone glucagon-like peptide-1 (GLP-1), modified with a side chain. It can be administered by subcutaneous injection or taken orally. It is sold under the brand names Ozempic (injectable) and Rybelsus (pill) for diabetes, and under the brand name Wegovy for weight loss.

Rabacfosadine, sold under the brand name Tanovea-CA1, is a guanine nucleotide analog used for the treatment of lymphoma in dogs. The drug was granted conditional approval by the U.S. Food and Drug Administration under application number 141-475 for use in treating canine lymphoma in December 2016 pending a full demonstration of effectiveness, and became the first drug to receive full approval for the treatment of canine lymphoma in July 2021.
Frunevetmab, sold under the brand name Solensia, is a medication used to treat pain associated with osteoarthritis in cats.