
Antiandrogens, also known as androgen antagonists or testosterone blockers, are a class of drugs that prevent androgens like testosterone and dihydrotestosterone (DHT) from mediating their biological effects in the body. They act by blocking the androgen receptor (AR) and/or inhibiting or suppressing androgen production. They can be thought of as the functional opposites of AR agonists, for instance androgens and anabolic steroids (AAS) like testosterone, DHT, and nandrolone and selective androgen receptor modulators (SARMs) like enobosarm. Antiandrogens are one of three types of sex hormone antagonists, the others being antiestrogens and antiprogestogens.

Ketoconazole, sold under the brand name Nizoral among others, is an antiandrogen, antifungal, and antiglucocorticoid medication used to treat a number of fungal infections. Applied to the skin it is used for fungal skin infections such as tinea, cutaneous candidiasis, pityriasis versicolor, dandruff, and seborrheic dermatitis. Taken by mouth it is a less preferred option and only recommended for severe infections when other agents cannot be used. Other uses include treatment of excessive male-patterned hair growth in women and Cushing's syndrome.

Finasteride, sold under the brand names Proscar and Propecia among others, is a medication used to treat pattern hair loss and benign prostatic hyperplasia (BPH) in men. It can also be used to treat excessive hair growth in women. It is usually taken orally but there are topical formulations for patients with hair loss, designed to minimize systemic exposure by acting specifically on hair follicles.

Bicalutamide, sold under the brand name Casodex among others, is an antiandrogen medication that is primarily used to treat prostate cancer. It is typically used together with a gonadotropin-releasing hormone (GnRH) analogue or surgical removal of the testicles to treat metastatic prostate cancer (mPC). To a lesser extent, it is used at high doses for locally advanced prostate cancer (LAPC) as a monotherapy without castration. Bicalutamide was also previously used as monotherapy to treat localized prostate cancer (LPC), but authorization for this use was withdrawn following unfavorable trial findings. Besides prostate cancer, bicalutamide is limitedly used in the treatment of excessive hair growth and scalp hair loss in women, as a puberty blocker and component of feminizing hormone therapy for transgender girls and women, to treat gonadotropin-independent early puberty in boys, and to prevent overly long-lasting erections in men. It is taken by mouth.
5α-Reductase inhibitors (5-ARIs), also known as dihydrotestosterone (DHT) blockers, are a class of medications with antiandrogenic effects which are used primarily in the treatment of enlarged prostate and scalp hair loss. They are also sometimes used to treat excess hair growth in women and as a component of hormone therapy for transgender women.
The management of hair loss, includes prevention and treatment of alopecia, baldness, and hair thinning, and regrowth of hair.

Flutamide, sold under the brand name Eulexin among others, is a nonsteroidal antiandrogen (NSAA) which is used primarily to treat prostate cancer. It is also used in the treatment of androgen-dependent conditions like acne, excessive hair growth, and high androgen levels in women. It is taken by mouth, usually three times per day.

Pattern hair loss (also known as androgenetic alopecia (AGA)) is a hair loss condition that primarily affects the top and front of the scalp. In male-pattern hair loss (MPHL), the hair loss typically presents itself as either a receding front hairline, loss of hair on the crown (vertex) of the scalp, or a combination of both. Female-pattern hair loss (FPHL) typically presents as a diffuse thinning of the hair across the entire scalp.
Non scarring hair loss, also known as noncicatricial alopecia is the loss of hair without any scarring being present. There is typically little inflammation and irritation, but hair loss is significant. This is in contrast to scarring hair loss during which hair follicles are replaced with scar tissue as a result of inflammation. Hair loss may be spread throughout the scalp (diffuse) or at certain spots (focal). The loss may be sudden or gradual with accompanying stress.
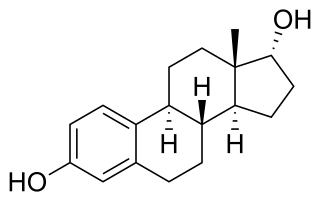
Alfatradiol, also known as 17α-estradiol and sold under the brand names Avicis, Avixis, Ell-Cranell Alpha, and Pantostin, is a weak estrogen and 5α-reductase inhibitor medication which is used topically in the treatment of pattern hair loss in men and women. It is a stereoisomer of the endogenous steroid hormone and estrogen 17β-estradiol.

RU-58642 is a nonsteroidal antiandrogen (NSAA) derived from nilutamide with very high affinity and selectivity for the androgen receptor (AR), which made it among the most potent and efficacious antiandrogens known at the time of its discovery. It was investigated for topical application for the treatment of androgenetic alopecia, but development did not proceed past initial trial stages, and it is now only used for scientific research into the AR.

RU-58841, also known as PSK-3841 or HMR-3841, is a nonsteroidal antiandrogen (NSAA) which was initially developed in the 1980s by Roussel Uclaf, the French pharmaceutical company from which it received its name. It was formerly under investigation by ProStrakan for potential use as a topical treatment for androgen-dependent conditions including acne, pattern hair loss, and excessive hair growth. The compound is similar in structure to the NSAA RU-58642 but contains a different side-chain. These compounds are similar in chemical structure to nilutamide, which is related to flutamide, bicalutamide, and enzalutamide, all of which are NSAAs similarly. RU-58841 can be synthesized either by building the hydantoin moiety or by aryl coupling to 5,5-dimethylhydantoin.

A nonsteroidal antiandrogen (NSAA) is an antiandrogen with a nonsteroidal chemical structure. They are typically selective and full or silent antagonists of the androgen receptor (AR) and act by directly blocking the effects of androgens like testosterone and dihydrotestosterone (DHT). NSAAs are used in the treatment of androgen-dependent conditions in men and women. They are the converse of steroidal antiandrogens (SAAs), which are antiandrogens that are steroids and are structurally related to testosterone.

Cioteronel is a nonsteroidal antiandrogen (NSAA) that was never marketed. It was under development between 1989 and 2001 for the topical treatment of androgenetic alopecia, and acne and for the oral treatment of benign prostatic hyperplasia; it reached phase III clinical trials for acne and phase II studies for androgenetic alopecia, but was ultimately discontinued due to poor efficacy.

Trimethyltrienolone (TMT), also known by its developmental code name R-2956 or RU-2956, is an antiandrogen medication which was never introduced for medical use but has been used in scientific research.

A steroidal antiandrogen (SAA) is an antiandrogen with a steroidal chemical structure. They are typically antagonists of the androgen receptor (AR) and act both by blocking the effects of androgens like testosterone and dihydrotestosterone (DHT) and by suppressing gonadal androgen production. SAAs lower concentrations of testosterone through simulation of the negative feedback inhibition of the hypothalamus. SAAs are used in the treatment of androgen-dependent conditions in men and women, and are also used in veterinary medicine for the same purpose. They are the converse of nonsteroidal antiandrogens (NSAAs), which are antiandrogens that are not steroids and are structurally unrelated to testosterone.
The medical uses of bicalutamide, a nonsteroidal antiandrogen (NSAA), include the treatment of androgen-dependent conditions and hormone therapy to block the effects of androgens. Indications for bicalutamide include the treatment of prostate cancer in men, skin and hair conditions such as acne, seborrhea, hirsutism, and pattern hair loss in women, high testosterone levels in women, hormone therapy in transgender women, as a puberty blocker to prevent puberty in transgender girls and to treat early puberty in boys, and the treatment of long-lasting erections in men. It may also have some value in the treatment of paraphilias and hypersexuality in men.

RU-59063 is a nonsteroidal androgen or selective androgen receptor modulator (SARM) which was first described in 1994 and was never marketed. It was originally thought to be a potent antiandrogen, but subsequent research found that it actually possesses dose-dependent androgenic activity, albeit with lower efficacy than dihydrotestosterone (DHT). The drug is an N-substituted arylthiohydantoin and was derived from the first-generation nonsteroidal antiandrogen (NSAA) nilutamide. The second-generation NSAAs enzalutamide, RD-162, and apalutamide were derived from RU-59063.

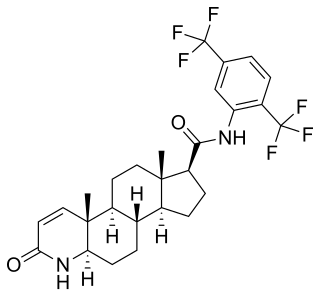
Pyrilutamide is a nonsteroidal antiandrogen (NSAA) – specifically, a selective high-affinity silent antagonist of the androgen receptor (AR) – which is under development by Suzhou Kintor Pharmaceuticals, inc., a subsidiary of Kintor Pharmaceutical Limited, for the potential treatment of androgenic alopecia As of October 2022, it is in phase 3 clinical trials for androgenic alopecia and phase 2 trials for acne.














