
Retinol, also called vitamin A1, is a fat-soluble vitamin in the vitamin A family that is found in food and used as a dietary supplement. Retinol or other forms of vitamin A are needed for vision, cellular development, maintenance of skin and mucous membranes, immune function and reproductive development. Dietary sources include fish, dairy products, and meat. As a supplement it is used to treat and prevent vitamin A deficiency, especially that which results in xerophthalmia. It is taken by mouth or by injection into a muscle. As an ingredient in skin-care products, it is used to reduce wrinkles and other effects of skin aging.
Isoflavones are substituted derivatives of isoflavone, a type of naturally occurring isoflavonoids, many of which act as phytoestrogens in mammals. Isoflavones occur in many plant species, but are especially high in soybeans.

Sida cordifolia is a perennial subshrub of the mallow family Malvaceae native to India. It has naturalized throughout the world, and is considered an invasive weed in Africa, Australia, the southern United States, Hawaiian Islands, New Guinea, and French Polynesia. The specific name, cordifolia, refers to the heart-shaped leaf.

Umbelliferone, also known as 7-hydroxycoumarin, hydrangine, skimmetine, and beta-umbelliferone, is a natural product of the coumarin family.

Ferulic acid is a hydroxycinnamic acid; it is an organic compound with the formula (CH3O)HOC6H3CH=CHCO2H. The name is derived from the genus Ferula, referring to the giant fennel (Ferula communis). Classified as a phenolic phytochemical, ferulic acid is an amber colored solid. Esters of ferulic acid are found in plant cell walls, covalently bonded to hemicellulose such as arabinoxylans. Salts and esters derived from ferulic acid are called ferulates.
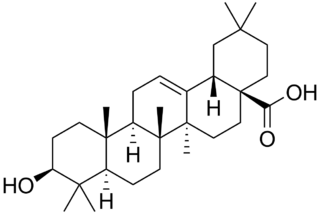
Oleanolic acid or oleanic acid is a naturally occurring pentacyclic triterpenoid related to betulinic acid. It is widely distributed in food and plants where it exists as a free acid or as an aglycone of triterpenoid saponins.

Galangin is a flavonol, a type of flavonoid.

Daphnin is a plant toxin with the chemical formula C15H16O9 and is one of the active compounds present in the Eurasian and North African genus Daphne of the Thymelaeaceae, a plant family with a predominantly Southern Hemisphere distribution with concentrations in Australia and tropical Africa.

Coronaridine, also known as 18-carbomethoxyibogamine, is an alkaloid found in Tabernanthe iboga and related species, including Tabernaemontana divaricata for which it was named.

In biochemistry, naturally occurring phenols are natural products containing at least one phenol functional group. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.

Verbascoside is a polyphenol glycoside in which the phenylpropanoid caffeic acid and the phenylethanoid hydroxytyrosol form an ester and an ether bond respectively, to the rhamnose part of a disaccharide, namely β-(3′,4′-dihydroxyphenyl)ethyl-O-α-L-rhamnopyranosyl(1→3)-β-D-(4-O-caffeoyl)-glucopyranoside.

Bufothionine is a sulfur-containing compound which is present in the bufotoxins secreted by the parotoid gland of certain toads of the genera Bufo and Chaunus. This specific compound can be found in the skin of certain species of toad such as the Asiatic Toad, Chaunus arunco, Chaunus crucifer, Chaunus spinulosus, and Chaunus arenarum.

Angelicin is the parent compound in a family of naturally occurring organic compounds known as the angular furanocoumarins. Structurally, it can be considered as benzapyra-2-one fused with a furan moiety in the 7,8-position. Angelicin is commonly found in certain Apiaceae and Fabaceae plant species such as Bituminaria bituminosa. It has a skin permeability coefficient (LogKp) of -2.46. The maximum absorption is observed at 300 nm. The 1HNMR spectrum is available; the infrared and mass spectra of angelicin can be found in this database. The sublimation of angelicin occurs at 120 °C and the pressure of 0.13 Pa. Angelicin is a coumarin.
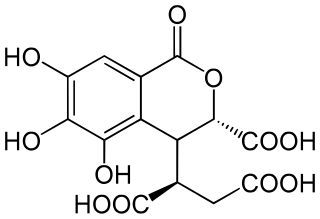
Chebulic acid is a phenolic compound isolated from the ripe fruits of Terminalia chebula.
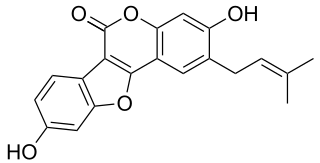
Psoralidin is a natural phenolic compound found in the seeds of Psoralea corylifolia.

Dianethole is a naturally occurring organic compound that is found in anise and fennel. It is a dimeric polymer of anethole. It has estrogenic activity, and along with anethole and photoanethole, may be responsible for the estrogenic effects of anise and fennel. These compounds bear resemblance to the estrogens stilbene and diethylstilbestrol, which may explain their estrogenic activity. In fact, it is said that diethylstilbestrol and related drugs were originally modeled after dianethole and photoanethole.

Drupanol is a naturally occurring phenol that has been isolated from the seeds of Psoralea drupaceae. Although drupanol is sometimes said to be the same compound as bakuchiol, the two compounds are in fact distinct; they have the same molecular formula and weight but different chemical structures and hence are structural isomers. Bakuchiol has been found to possess antiandrogenic activity in vitro.

The flora of Nepal is one of the richest in the world due to the diverse climate, topology and geography of the country. Research undertaken in the late 1970s and early 1980s documented 5067 species of which 5041 were angiosperms and the remaining 26 species were gymnosperms. The Terai area has hardwood, bamboo, palm, and sal trees. Notable plants include the garden angelica, Luculia gratissima, Meconopsis villosa, and Persicaria affinis. However, according to ICOMOS checklist, in the protected sites, there are 2,532 species of vascular plants under 1,034 genera and 199 families. The variation in figures is attributed to inadequate floral coverage filed studies. Some of the plants contain medicinal values. It contains certain chemical which is used to heal wound by There are 400 species of vascular plants which are endemic to Nepal. Of these, two in particular are orchids Pleione coronaria and Oreorchis porphyranthes. The most popular endemic plant of Nepal is rhododendron (arboreum) which in Nepali language is called lali guras.
John Massa Kasenene is a botanical and environmental ecologist, academic, scientist and academic administrator in Uganda. From 4 October 2022, he serves as the substantive Deputy Vice Chancellor of the Mountains of the Moon University (MMU), at that time, the tenth public university in the country.

















