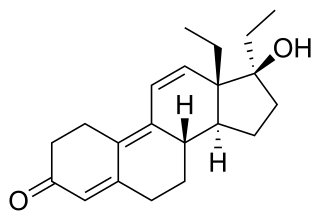
Tetrahydrogestrinone (THG), known by the nickname The Clear, is a synthetic and orally active anabolic–androgenic steroid (AAS) which was never marketed for medical use. It was developed by Patrick Arnold and was used by a number of high-profile athletes such as Marion Jones, Barry Bonds, and Dwain Chambers.
Methyltestosterone, sold under the brand names Android, Metandren, and Testred among others, is an androgen and anabolic steroid (AAS) medication which is used in the treatment of low testosterone levels in men, delayed puberty in boys, at low doses as a component of menopausal hormone therapy for menopausal symptoms like hot flashes, osteoporosis, and low sexual desire in women, and to treat breast cancer in women. It is taken by mouth or held in the cheek or under the tongue.

Nandrolone, also known as 19-nortestosterone, is an endogenous androgen which exists in the male body at a ratio of 1:50 compared to testosterone. It is also an anabolic steroid (AAS) which is medically used in the form of esters such as nandrolone decanoate and nandrolone phenylpropionate. Nandrolone esters are used in the treatment of anemias, cachexia, osteoporosis, breast cancer, and for other indications. They are not used by mouth and instead are given by injection into muscle or fat.

Metenolone, or methenolone, is an androgen and anabolic steroid (AAS) which is used in the form of esters such as metenolone acetate and metenolone enanthate. Metenolone esters are used mainly in the treatment of anemia due to bone marrow failure. Metenolone acetate is taken by mouth, while metenolone enanthate is given by injection into muscle.

Nandrolone decanoate, sold under the brand name ROLON among others, is an androgen and anabolic steroid (AAS) medication which is used primarily in the treatment of anemias and wasting syndromes, as well as osteoporosis in menopausal women. It is given by injection into muscle or fat once every one to four weeks.
Oxymetholone, sold under the brand names Anadrol and Anapolon among others, is an androgen and anabolic steroid (AAS) medication which is used primarily in the treatment of anemia. It is also used to treat osteoporosis, HIV/AIDS wasting syndrome, and to promote weight gain and muscle growth in certain situations. It is taken by mouth.

Norethandrolone, sold under the brand names Nilevar and Pronabol among others, is an androgen and anabolic steroid (AAS) medication which has been used to promote muscle growth and to treat severe burns, physical trauma, and aplastic anemia but has mostly been discontinued. It is still available for use in France however. It is taken by mouth.

Mesterolone, sold under the brand name Proviron among others, is an androgen and anabolic steroid (AAS) medication which is used mainly in the treatment of low testosterone levels. It has also been used to treat male infertility, although this use is controversial. It is taken by mouth.

Fluoxymesterone, sold under the brand names Halotestin and Ultandren among others, is an androgen and anabolic steroid (AAS) medication which is used in the treatment of low testosterone levels in men, delayed puberty in boys, breast cancer in women, and anemia. It is taken by mouth.

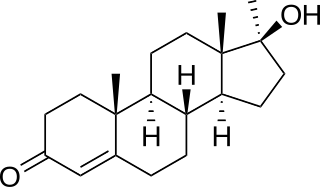
Metribolone is a synthetic and orally active anabolic–androgenic steroid (AAS) and a 17α-alkylated nandrolone (19-nortestosterone) derivative which was never marketed for medical use but has been widely used in scientific research as a hot ligand in androgen receptor (AR) ligand binding assays (LBAs) and as a photoaffinity label for the AR. More precisely, metribolone is the 17α-methylated derivative of trenbolone. It was investigated briefly for the treatment of advanced breast cancer in women in the late 1960s and early 1970s, but was found to produce signs of severe hepatotoxicity at very low dosages, and its development was subsequently discontinued.

Anabolic steroids, also known as anabolic-androgenic steroids (AAS), are a class of drugs that are structurally related to testosterone, the main male sex hormone, and produce effects by binding to the androgen receptor. Anabolic steroids have a number of medical uses, but are also used by athletes to increase muscle size, strength, and performance.

Normethandrone, also known as methylestrenolone or methylnortestosterone and sold under the brand name Metalutin among others, is a progestin and androgen/anabolic steroid (AAS) medication which is used in combination with an estrogen in the treatment of amenorrhea and menopausal symptoms in women. It is taken by mouth.
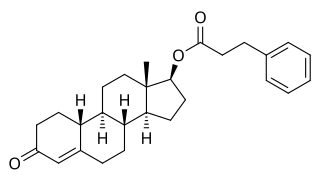
Nandrolone phenylpropionate (NPP), or nandrolone phenpropionate, sold under the brand name Durabolin among others, is an androgen and anabolic steroid (AAS) medication which has been used primarily in the treatment of breast cancer and osteoporosis in women. It is given by injection into muscle once every week. Although it was widely used in the past, the drug has mostly been discontinued and hence is now mostly no longer available.
A 17α-alkylated anabolic steroid is a synthetic anabolic–androgenic steroid (AAS) that features an alkyl group, specifically a methyl or ethyl group, at the C17α position. Unlike many other AAS, 17α-alkylated AAS are orally active and do not require intramuscular injection. However, they uniquely possess a high potential for hepatotoxicity, which simultaneously limits their use. In addition, some have a high risk of gynecomastia due to uniquely high estrogenic activity, although this does not apply to 17α-alkylated AAS that are also 4,5α-reduced or 19-demethylated. The prototypical example of a 17α-alkylated AAS is methyltestosterone (17α-methyltestosterone).

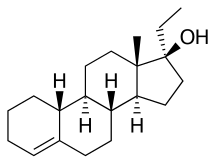
Ethyltestosterone, or 17α-ethyltestosterone, also known as 17α-ethylandrost-4-en-17β-ol-3-one or 17α-pregn-4-en-17-ol-3-one, is a synthetic, orally active anabolic–androgenic steroid (AAS) of the 17α-alkylated group related to methyltestosterone which was never marketed. Like methyltestosterone, ethyltestosterone is the parent compound of many AAS. Derivatives of ethyltestosterone include norethandrolone, ethylestrenol (ethylnandrol), norboletone, ethyldienolone, tetrahydrogestrinone, bolenol (ethylnorandrostenol), and propetandrol.
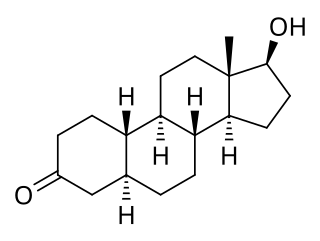
5α-Dihydronandrolone is a naturally occurring anabolic–androgenic steroid (AAS) and a 5α-reduced derivative of nandrolone (19-nortestosterone). It is a major metabolite of nandrolone and is formed from it by the actions of the enzyme 5α-reductase analogously to the formation of dihydrotestosterone (DHT) from testosterone.

The structure–activity relationships (SAR) of anabolic steroids (AAS) have been extensively studied.