Luteinizing hormone is a hormone produced by gonadotropic cells in the anterior pituitary gland. The production of LH is regulated by gonadotropin-releasing hormone (GnRH) from the hypothalamus. In females, an acute rise of LH known as an LH surge, triggers ovulation and development of the corpus luteum. In males, where LH had also been called interstitial cell–stimulating hormone (ICSH), it stimulates Leydig cell production of testosterone. It acts synergistically with follicle-stimulating hormone (FSH).

Follicle-stimulating hormone (FSH) is a gonadotropin, a glycoprotein polypeptide hormone. FSH is synthesized and secreted by the gonadotropic cells of the anterior pituitary gland and regulates the development, growth, pubertal maturation, and reproductive processes of the body. FSH and luteinizing hormone (LH) work together in the reproductive system.
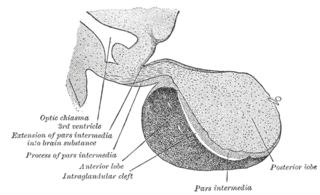
A major organ of the endocrine system, the anterior pituitary is the glandular, anterior lobe that together with the posterior lobe makes up the pituitary gland (hypophysis) which, in humans, is located at the base of the brain, protruding off the bottom of the hypothalamus.
Gonadotropic cells are endocrine cells in the anterior pituitary that produce the gonadotropins, such as the follicle-stimulating hormone (FSH) and luteinizing hormone (LH). Release of FSH and LH by gonadotropes is regulated by gonadotropin-releasing hormone (GnRH) from the hypothalamus.
Gonadotropins are glycoprotein hormones secreted by gonadotropic cells of the anterior pituitary of vertebrates. This family includes the mammalian hormones follicle-stimulating hormone (FSH) and luteinizing hormone (LH), the placental/chorionic gonadotropins, human chorionic gonadotropin (hCG) and equine chorionic gonadotropin (eCG), as well as at least two forms of fish gonadotropins. These hormones are central to the complex endocrine system that regulates normal growth, sexual development, and reproductive function. LH and FSH are secreted by the anterior pituitary gland, while hCG and eCG are secreted by the placenta in pregnant humans and mares, respectively. The gonadotropins act on the gonads, controlling gamete and sex hormone production.
Gonadarche refers to the earliest gonadal changes of puberty. In response to pituitary gonadotropins, the ovaries in females and the testes in males begin to grow and increase the production of the sex steroids, especially estradiol and testosterone. The ovary and testis have receptors, follicle cells and leydig cells, respectively, where gonadotropins bind to stimulate the maturation of the gonads and secretion of estrogen and testosterone. Certain disorders can result in changes to timing or nature of these processes.

The hypothalamic–pituitary–gonadal axis refers to the hypothalamus, pituitary gland, and gonadal glands as if these individual endocrine glands were a single entity. Because these glands often act in concert, physiologists and endocrinologists find it convenient and descriptive to speak of them as a single system.

A gonadotropin-releasing hormone agonist is a type of medication which affects gonadotropins and sex hormones. They are used for a variety of indications including in fertility medicine and to lower sex hormone levels in the treatment of hormone-sensitive cancers such as prostate cancer and breast cancer, certain gynecological disorders like heavy periods and endometriosis, high testosterone levels in women, early puberty in children, as a part of transgender hormone therapy, and to delay puberty in transgender youth among other uses. It is also used in the suppression of spontaneous ovulation as part of controlled ovarian hyperstimulation, an essential component in IVF. GnRH agonists are given by injections into fat, as implants placed into fat, and as nasal sprays.
The gonadotropin-releasing hormone receptor (GnRHR), also known as the luteinizing hormone releasing hormone receptor (LHRHR), is a member of the seven-transmembrane, G-protein coupled receptor (GPCR) family. It is the receptor of gonadotropin-releasing hormone (GnRH). The GnRHR is expressed on the surface of pituitary gonadotrope cells as well as lymphocytes, breast, ovary, and prostate.

Kisspeptins are proteins encoded by the KISS1 gene in humans. Kisspeptins are ligands of the G-protein coupled receptor, GPR54. Kiss1 was originally identified as a human metastasis suppressor gene that has the ability to suppress melanoma and breast cancer metastasis. Kisspeptin-GPR54 signaling has an important role in initiating secretion of gonadotropin-releasing hormone (GnRH) at puberty, the extent of which is an area of ongoing research. Gonadotropin-releasing hormone is released from the hypothalamus to act on the anterior pituitary triggering the release of luteinizing hormone (LH), and follicle stimulating hormone (FSH). These gonadotropic hormones lead to sexual maturation and gametogenesis. Disrupting GPR54 signaling can cause hypogonadotrophic hypogonadism in rodents and humans. The Kiss1 gene is located on chromosome 1. It is transcribed in the brain, adrenal gland, and pancreas.

Neurokinin B (NKB) belongs in the family of tachykinin peptides. Neurokinin B is implicated in a variety of human functions and pathways such as the secretion of gonadotropin-releasing hormone. Additionally, NKB is associated with pregnancy in females and maturation in young adults. Reproductive function is highly dependent on levels of both neurokinin B and also the G-protein coupled receptor ligand kisspeptin. The first NKB studies done attempted to resolve why high levels of the peptide may be implicated in pre-eclampsia during pregnancy. NKB, kisspeptin, and dynorphin together are found in the arcuate nucleus (ARC) known as the KNDy subpopulation. This subpopulation is targeted by many steroid hormones and works to form a network that feeds back to GnRH pulse generator.

The KiSS1-derived peptide receptor is a G protein-coupled receptor which binds the peptide hormone kisspeptin (metastin). Kisspeptin is encoded by the metastasis suppressor gene KISS1, which is expressed in a variety of endocrine and gonadal tissues. Activation of the kisspeptin receptor is linked to the phospholipase C and inositol trisphosphate second messenger cascades inside the cell.
Hypothalamic–pituitary hormones are hormones that are produced by the hypothalamus and pituitary gland. Although the organs in which they are produced are relatively small, the effects of these hormones cascade throughout the body. They can be classified as a hypothalamic–pituitary axis of which the adrenal, gonadal, thyroid, somatotropic, and prolactin axes are branches.
Hypogonadotropic hypogonadism (HH), is due to problems with either the hypothalamus or pituitary gland affecting the hypothalamic-pituitary-gonadal axis. Hypothalamic disorders result from a deficiency in the release of gonadotropic releasing hormone (GnRH), while pituitary gland disorders are due to a deficiency in the release of gonadotropins from the anterior pituitary. GnRH is the central regulator in reproductive function and sexual development via the HPG axis. GnRH is released by GnRH neurons, which are hypothalamic neuroendocrine cells, into the hypophyseal portal system acting on gonadotrophs in the anterior pituitary. The release of gonadotropins, LH and FSH, act on the gonads for the development and maintenance of proper adult reproductive physiology. LH acts on Leydig cells in the male testes and theca cells in the female. FSH acts on Sertoli cells in the male and follicular cells in the female. Combined this causes the secretion of gonadal sex steroids and the initiation of folliculogenesis and spermatogenesis. The production of sex steroids forms a negative feedback loop acting on both the anterior pituitary and hypothalamus causing a pulsatile secretion of GnRH. GnRH neurons lack sex steroid receptors and mediators such as kisspeptin stimulate GnRH neurons for pulsatile secretion of GnRH.

GnRH neurons, or gonadotropin-releasing hormone expressing neurons, are the cells in the brain that control the release of reproductive hormones from the pituitary. These brain cells control reproduction by secreting GnRH into the hypophyseal portal capillary bloodstream, so are sometimes referred to as “sex neurons”. This small capillary network carries GnRH to the anterior pituitary, causing release of luteinizing hormone (LH) and follicle stimulating hormone (FSH) into the wider bloodstream. When GnRH neurons change their pattern of release from the juvenile to the adult pattern of GnRH secretion, puberty is initiated. Failure of GnRH neurons to form the proper connections, or failure to successfully stimulate the pituitary with GnRH, means that puberty is not initiated. These disruptions to the GnRH system cause reproductive disorders like hypogonadotropic hypogonadism or Kallmann Syndrome.
Pulsatile secretion is a biochemical phenomenon observed in a wide variety of cell and tissue types, in which chemical products are secreted in a regular temporal pattern. The most common cellular products observed to be released in this manner are intercellular signaling molecules such as hormones or neurotransmitters. Examples of hormones that are secreted pulsatilely include insulin, thyrotropin, TRH, gonadotropin-releasing hormone (GnRH) and growth hormone (GH). In the nervous system, pulsatility is observed in oscillatory activity from central pattern generators. In the heart, pacemakers are able to work and secrete in a pulsatile manner. A pulsatile secretion pattern is critical to the function of many hormones in order to maintain the delicate homeostatic balance necessary for essential life processes, such as development and reproduction. Variations of the concentration in a certain frequency can be critical to hormone function, as evidenced by the case of GnRH agonists, which cause functional inhibition of the receptor for GnRH due to profound downregulation in response to constant (tonic) stimulation. Pulsatility may function to sensitize target tissues to the hormone of interest and upregulate receptors, leading to improved responses. This heightened response may have served to improve the animal's fitness in its environment and promote its evolutionary retention.

A GnRH modulator, or GnRH receptor modulator, also known as an LHRH modulator or LHRH receptor modulator, is a type of medication which modulates the GnRH receptor, the biological target of the hypothalamic hormone gonadotropin-releasing hormone. They include GnRH agonists and GnRH antagonists. These medications may be GnRH analogues like leuprorelin and cetrorelix – peptides that are structurally related to GnRH – or small-molecules like elagolix and relugolix, which are structurally distinct from and unrelated to GnRH analogues.
Gonadotropin surge-attenuating factor (GnSAF) is a nonsteroidal ovarian hormone produced by the granulosa cells of small antral ovarian follicles in females. GnSAF is involved in regulating the secretion of luteinizing hormone (LH) from the anterior pituitary and the ovarian cycle. During the early to mid-follicular phase of the ovarian cycle, GnSAF acts on the anterior pituitary to attenuate LH release, limiting the secretion of LH to only basal levels. At the transition between follicular and luteal phase, GnSAF bioactivity declines sufficiently to permit LH secretion above basal levels, resulting in the mid-cycle LH surge that initiates ovulation. In normally ovulating women, the LH surge only occurs when the oocyte is mature and ready for extrusion. GnSAF bioactivity is responsible for the synchronised, biphasic nature of LH secretion.
Kisspeptin, neurokinin B, and dynorphin (KNDy) neurons are neurons in the hypothalamus of the brain that are central to the hormonal control of reproduction.
Gonadotropin-inhibitory hormone (GnIH) is a RFamide-related peptide coded by the NPVF gene in mammals.