Cholecystokinin is a peptide hormone of the gastrointestinal system responsible for stimulating the digestion of fat and protein. Cholecystokinin, formerly called pancreozymin, is synthesized and secreted by enteroendocrine cells in the duodenum, the first segment of the small intestine. Its presence causes the release of digestive enzymes and bile from the pancreas and gallbladder, respectively, and also acts as a hunger suppressant.

Gastrin is a peptide hormone that stimulates secretion of gastric acid (HCl) by the parietal cells of the stomach and aids in gastric motility. It is released by G cells in the pyloric antrum of the stomach, duodenum, and the pancreas.
Bombesin is a 14-amino acid peptide originally isolated from the skin of the European fire-bellied toad by Vittorio Erspamer et al. and named after its source. It has two known homologs in mammals called neuromedin B and gastrin-releasing peptide. It stimulates gastrin release from G cells. It activates three different G-protein-coupled receptors known as BBR1, -2, and -3. It also activates these receptors in the brain. Together with cholecystokinin, it is the second major source of negative feedback signals that stop eating behaviour.

Opioid peptides or opiate peptides are peptides that bind to opioid receptors in the brain; opiates and opioids mimic the effect of these peptides. Such peptides may be produced by the body itself, for example endorphins. The effects of these peptides vary, but they all resemble those of opiates. Brain opioid peptide systems are known to play an important role in motivation, emotion, attachment behaviour, the response to stress and pain, control of food intake, and the rewarding effects of alcohol and nicotine.
Neuromodulation is the physiological process by which a given neuron uses one or more chemicals to regulate diverse populations of neurons. Neuromodulators typically bind to metabotropic, G-protein coupled receptors (GPCRs) to initiate a second messenger signaling cascade that induces a broad, long-lasting signal. This modulation can last for hundreds of milliseconds to several minutes. Some of the effects of neuromodulators include: altering intrinsic firing activity, increasing or decreasing voltage-dependent currents, altering synaptic efficacy, increasing bursting activity and reconfigurating synaptic connectivity.
Neuromedin B (NMB) is a bombesin-related peptide in mammals. It was originally purified from pig spinal cord, and later shown to be present in human central nervous system and gastrointestinal tract.
H1299, also known as NCI-H1299 or CRL-5803, is a human non-small cell lung carcinoma cell line derived from the lymph node, which is widely used in research.

The bombesin receptor subtype 3 also known as BRS-3 or BB3 is a protein which in humans is encoded by the BRS3 gene.

The neuromedin B receptor (NMBR), now known as BB1 is a G protein-coupled receptor whose endogenous ligand is neuromedin B. In humans, this protein is encoded by the NMBR gene.

The gastrin-releasing peptide receptor (GRPR), now properly known as BB2 is a G protein-coupled receptor whose endogenous ligand is gastrin releasing peptide. In humans it is highly expressed in the pancreas and is also expressed in the stomach, adrenal cortex and brain.

The prolactin-releasing peptide receptor (PrRPR) also known as G-protein coupled receptor 10 (GPR10) is a protein that in humans is encoded by the PRLHR gene.

Somatostatin receptor type 5 is a protein that in humans is encoded by the SSTR5 gene.

Neuropeptide FF receptor 2, also known as NPFF2 is a human protein encoded by the NPFFR2 gene.
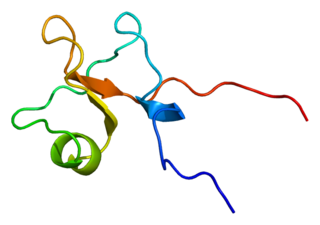
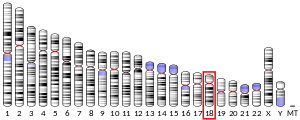
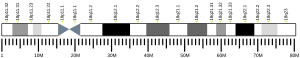
Trefoil factor 3 is a protein that in humans is encoded by the TFF3 gene.

Tachykinin-3 is a protein that in humans is encoded by the TAC3 gene.

DNA-directed RNA polymerase III subunit RPC9 is an enzyme that in humans is encoded by the CRCP gene.

Preprotachykinin-1,, is a precursor protein that in humans is encoded by the TAC1 gene.
Bombesin-like peptides comprise a large family of peptides which were initially isolated from amphibian skin, where they stimulate smooth muscle contraction. They were later found to be widely distributed in mammalian neural and endocrine cells.
Pro-gastrin-releasing-peptide, also known as Pro-GRP, is a gastrin-releasing peptide (GRP) precursor, a neurotransmitter that belongs to the bombesin-related neuromedin B family. GRP stimulates the secretion of gastrin in order to increase the acidity of the gastric acid. Pro-GRP is a peptide composed of 125 amino acids, expressed in the nervous system and digestive tract. It is different from progastrin, consisting of 80 amino acids, precursor of gastrin in its intracellular version and oncogene in its extracellular version (hPG80).
Progastrin is an 80-amino acid intracellular protein and the precursor of gastrin, a gastrointestinal hormone produced by G cells in the gastric antrum. The main function of gastrin is to regulate acid secretion. During digestion, only gastrin is released into the bloodstream and stimulates the secretion of hydrochloric acid in the stomach as well as pancreatic digestive enzymes. In humans, progastrin is encoded by the GAST gene. Progastrin is expressed primarily in stomach tissue.