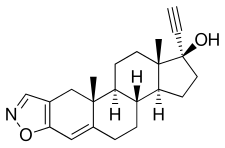
Antiandrogens, also known as androgen antagonists or testosterone blockers, are a class of drugs that prevent androgens like testosterone and dihydrotestosterone (DHT) from mediating their biological effects in the body. They act by blocking the androgen receptor (AR) and/or inhibiting or suppressing androgen production. They can be thought of as the functional opposites of AR agonists, for instance androgens and anabolic steroids (AAS) like testosterone, DHT, and nandrolone and selective androgen receptor modulators (SARMs) like enobosarm. Antiandrogens are one of three types of sex hormone antagonists, the others being antiestrogens and antiprogestogens.
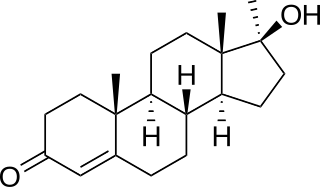
Methyltestosterone, sold under the brand names Android, Metandren, and Testred among others, is an androgen and anabolic steroid (AAS) medication which is used in the treatment of low testosterone levels in men, delayed puberty in boys, at low doses as a component of menopausal hormone therapy for menopausal symptoms like hot flashes, osteoporosis, and low sexual desire in women, and to treat breast cancer in women. It is taken by mouth or held in the cheek or under the tongue.

Sex hormone-binding globulin (SHBG) or sex steroid-binding globulin (SSBG) is a glycoprotein that binds to androgens and estrogens. When produced by the Sertoli cells in the seminiferous tubules of the testis, it is called androgen-binding protein (ABP).

Norgestimate, sold under the brand names Ortho Tri-Cyclen and Previfem among others, is a progestin medication which is used in birth control pills for women and in menopausal hormone therapy. The medication is available in combination with an estrogen and is not available alone. It is taken by mouth.
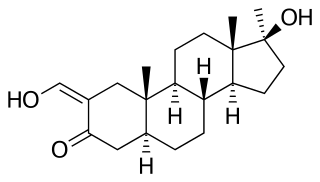
Oxymetholone, sold under the brand names Anadrol and Anapolon among others, is an androgen and anabolic steroid (AAS) medication which is used primarily in the treatment of anemia. It is also used to treat osteoporosis, HIV/AIDS wasting syndrome, and to promote weight gain and muscle growth in certain situations. It is taken by mouth.

Ethylestrenol, also known as ethyloestrenol or ethylnandrol and sold under the brand names Maxibolin and Orabolin among others, is an androgen and anabolic steroid (AAS) medication which has been used in the past for a variety of indications such as to promote weight gain and to treat anemia and osteoporosis but has been discontinued for use in humans. It is still available for veterinary use in Australia and New Zealand however. It is taken by mouth.

Gestrinone, sold under the brand names Dimetrose and Nemestran among others, is a medication which is used in the treatment of endometriosis. It has also been used to treat other conditions such as uterine fibroids and heavy menstrual bleeding and has been investigated as a method of birth control. Gestrinone is used alone and is not formulated in combination with other medications. It is taken by mouth or in through the vagina.

Norethisterone, also known as norethindrone and sold under many brand names, is a progestin medication used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication is available in both low-dose and high-dose formulations and both alone and in combination with an estrogen. It is used by mouth or, as norethisterone enanthate, by injection into muscle.

Mesterolone, sold under the brand name Proviron among others, is an androgen and anabolic steroid (AAS) medication which is used mainly in the treatment of low testosterone levels. It has also been used to treat male infertility, although this use is controversial. It is taken by mouth.

Ethisterone, also known as ethinyltestosterone, pregneninolone, and anhydrohydroxyprogesterone and formerly sold under the brand names Proluton C and Pranone among others, is a progestin medication which was used in the treatment of gynecological disorders but is now no longer available. It was used alone and was not formulated in combination with an estrogen. The medication is taken by mouth.

Trestolone, also known as 7α-methyl-19-nortestosterone (MENT), is an experimental androgen/anabolic steroid (AAS) and progestogen medication which has been under development for potential use as a form of hormonal birth control for men and in androgen replacement therapy for low testosterone levels in men but has never been marketed for medical use. It is given as an implant that is placed into fat. As trestolone acetate, an androgen ester and prodrug of trestolone, the medication can also be given by injection into muscle.

Noretynodrel, or norethynodrel, sold under the brand name Enovid among others, is a progestin medication which was previously used in birth control pills and in the treatment of gynecological disorders but is now no longer marketed. It was available both alone and in combination with an estrogen. The medication is taken by mouth.
An antigonadotropin is a drug which suppresses the activity and/or downstream effects of one or both of the gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH). This results in an inhibition of the hypothalamic-pituitary-gonadal (HPG) axis, and thus a decrease in the levels of the androgen, estrogen, and progestogen sex steroids in the body. Antigonadotropins also inhibit ovulation in women and spermatogenesis in men. They are used for a variety of purposes, including for the hormonal birth control, treatment of hormonally-sensitive cancers, to delay precocious puberty and puberty in transgender youth, as a form of chemical castration to reduce the sex drives of individuals with hypersexuality or pedophilia, and to treat estrogen-associated conditions in women such as menorrhagia and endometriosis, among others. High-dose antigonadotropin therapy has been referred to as medical castration.

Segesterone acetate (SGA), sold under the brand names Nestorone, Elcometrine, and Annovera, is a progestin medication which is used in birth control and in the treatment of endometriosis in the United States, Brazil, and other South American countries. It is available both alone and in combination with an estrogen. It is not effective by mouth and must be given by other routes, most typically as a vaginal ring or implant that is placed into fat.
A steroidogenesis inhibitor, also known as a steroid biosynthesis inhibitor, is a type of drug which inhibits one or more of the enzymes that are involved in the process of steroidogenesis, the biosynthesis of endogenous steroids and steroid hormones. They may inhibit the production of cholesterol and other sterols, sex steroids such as androgens, estrogens, and progestogens, corticosteroids such as glucocorticoids and mineralocorticoids, and neurosteroids. They are used in the treatment of a variety of medical conditions that depend on endogenous steroids.

A steroidal antiandrogen (SAA) is an antiandrogen with a steroidal chemical structure. They are typically antagonists of the androgen receptor (AR) and act both by blocking the effects of androgens like testosterone and dihydrotestosterone (DHT) and by suppressing gonadal androgen production. SAAs lower concentrations of testosterone through simulation of the negative feedback inhibition of the hypothalamus. SAAs are used in the treatment of androgen-dependent conditions in men and women, and are also used in veterinary medicine for the same purpose. They are the converse of nonsteroidal antiandrogens (NSAAs), which are antiandrogens that are not steroids and are structurally unrelated to testosterone.

5α-Dihydronorethisterone is a major active metabolite of norethisterone (norethindrone). Norethisterone is a progestin with additional weak androgenic and estrogenic activity. 5α-DHNET is formed from norethisterone by 5α-reductase in the liver and other tissues.
A sex-hormonal agent, also known as a sex-hormone receptor modulator, is a type of hormonal agent which specifically modulates the effects of sex hormones and of their biological targets, the sex hormone receptors. The sex hormones include androgens such as testosterone, estrogens such as estradiol, and progestogens such as progesterone. Sex-hormonal agents may be either steroidal or nonsteroidal in chemical structure and may serve to either enhance, inhibit, or have mixed effects on the function of the sex hormone systems.

5α-Dihydroethisterone is an active metabolite of the formerly clinically used but now-discontinued progestin ethisterone and the experimental and never-marketed hormonal antineoplastic agent ethynylandrostanediol (HE-3235). Its formation from its parent drugs is catalyzed by 5α-reductase in tissues that express the enzyme in high amounts like the liver, skin, hair follicles, and prostate gland. 5α-DHET has significant affinity for steroid hormone receptors and may contribute importantly to the activities of its parent drugs.