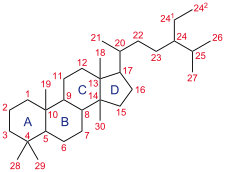
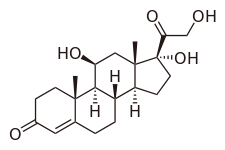
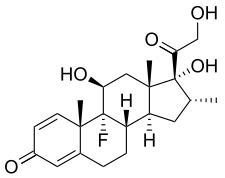
Triamcinolone acetonide, sold under the brand name Kenalog among others, is a synthetic corticosteroid medication used topically to treat various skin conditions, to relieve the discomfort of mouth sores, and by injection into joints to treat various joint conditions. It is also injected into lesions to treat inflammation in some parts of the body, particularly the skin. In nasal spray form, it is used to treat allergic rhinitis. It is used for the treatment of macular edema associated with uveitis. It is a more potent derivative of triamcinolone, and is about eight times as potent as prednisone.

Fluorometholone, also known as 6α-methyl-9α-fluoro-11β,17α-dihydroxypregna-1,4-diene-3,20-dione, is a synthetic glucocorticoid which is used in the treatment of inflammatory eye diseases. The C17α acetate ester, fluorometholone acetate, is also a glucocorticoid and is used for similar indications.

Medroxyprogesterone (MP), is a progestin which is not used medically. A derivative, medroxyprogesterone acetate (MPA), is used as a medication in humans, and is far more widely known in comparison. Medroxyprogesterone is sometimes used as a synonym for medroxyprogesterone acetate, and what is almost always being referred to when the term is used is MPA and not medroxyprogesterone.
The molecular formula C22H29FO4 (molar mass: 376.46 g/mol) may refer to:
The molecular formula C21H27ClO3 (molar mass: 362.89028 g/mol, exact mass: 362.1649 u) may refer to:

Chloroprednisone is a topical glucocorticoid first reported in 1960. It is a chlorinated derivative of prednisone. The acetate ester prodrug, chloroprednisone 21-acetate, was sold under the brand name Topilan as an anti-inflammatory agent.
Haloprogesterone, sold under the brand name Prohalone, is a progestin medication which was previously marketed by Ayerst but is now no longer available.
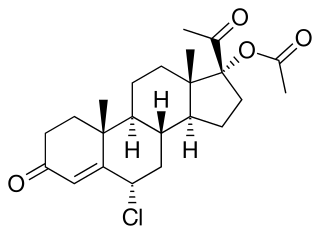
Hydromadinone acetate, also known as chloroacetoxyprogesterone (CAP), as well as 6α-chloro-17α-acetoxyprogesterone or 6α-chloro-17α-acetoxypregn-4-ene-3,20-dione, is a steroidal progestin of the 17α-hydroxyprogesterone group that was never marketed. It is the C17α acetate ester of hydromadinone, which, similarly, was never marketed.

Flugestone acetate (FGA), sold under the brand name Cronolone among others, is a progestin medication which is used in veterinary medicine.

Cismadinone (INN), also known as 6α-chloro-17α-hydroxypregna-1,4-diene-3,20-dione or 6α-chloro-δ1-dehydro-17α-hydroxyprogesterone, is a steroidal progestin closely related to the 17α-hydroxyprogesterone derivatives that was never marketed. An acetylated form, cismadinone acetate, also exists, but similarly to cismadinone, was never marketed.

Cismadinone acetate, also known as 6α-chloro-δ1-dehydro-17α-acetoxyprogesterone or as 6α-chloro-17α-acetoxypregna-1,4-diene-3,20-dione, is a steroidal progestin related to the 17α-hydroxyprogesterone derivatives which was never marketed. It is the acetylated form of cismadinone, which is also a progestin but, similarly to cismadinone acetate, was never marketed.

Clomegestone (INN), or clomagestone, also known as 6-chloro-17α-hydroxy-16α-methylpregna-4,6-diene-3,20-dione, is a steroidal progestin of the 17α-hydroxyprogesterone group that was never marketed. An acetate ester, clomegestone acetate, also exists, and similarly was never marketed.
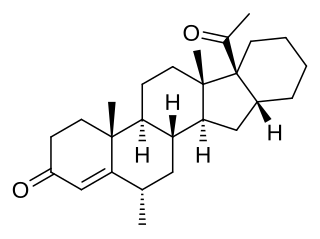
Mecigestone, also known as pentarane B, as well as 6α-methyl-16α,17α-cyclohexanoprogesterone, 6α-methylcyclohexano[1',2';16,17]pregn-4-ene-3,20-dione, or 17α-acetyl-6α-methyl-16β,24-cyclo-21-norchol-4-en-3-one, is a steroidal progestin that was developed by the Zelinskii Institute of Organic Chemistry of the Russian Academy of Sciences and has been proposed for clinical use as a progestogen but has not been marketed. It is the 6α-methylated analogue of pentarane A, which is also known as D'6-pentarane or pregna-D'6-pentarane.

21-Deoxycortisone, also known as 21-desoxycortisone, 11-keto-17α-hydroxyprogesterone, or 17α-hydroxypregn-4-ene-3,11,20-trione, is a naturally occurring, endogenous steroid and minor intermediate and metabolite in corticosteroid metabolism. It is related to 21-deoxycortisol (11β,17α-dihydroxyprogesterone) and is reversibly formed from it by 11β-hydroxysteroid dehydrogenase, analogously to the reversible formation of cortisone from cortisol. 21-Deoxycortisone can be transformed into cortisone by 21-hydroxylase.
Deoxycortisone, or desoxycortisone, may refer to:
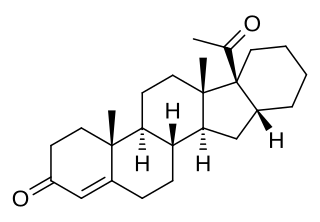
Pentarane A, also known as D'6-pentarane or pregna-D'6-pentarane, as well as 16α,17α-cyclohexanoprogesterone, 16α,17α-tetramethylenepregn-4-ene-3,20-dione, or 17α-acetyl-16β,24-cyclo-21-norchol-4-en-3-one, is a steroidal progestin that was developed by the Zelinskii Institute of Organic Chemistry of the Russian Academy of Sciences and was never marketed. The 6α-methylated analogue of pentarane A is known as mecigestone or as pentarane B.

Megestrol caproate, abbreviated as MGC, is a progestin medication which was never marketed. It was developed in Russia in 2002. In animals, MGC shows 10-fold higher progestogenic activity compared to progesterone when both are administered via subcutaneous injection. In addition, MGC has no androgenic, anabolic, or estrogenic activity. The medication was suggested as a potential contraceptive and therapeutic agent.

16-Methylene-17α-hydroxyprogesterone acetate is a progestin of the 17α-hydroxyprogesterone group which was never marketed. Given orally, it shows about 2.5-fold the progestogenic activity of parenteral progesterone in animal bioassays. It is a parent compound of the following clinically used progestins: