
Estropipate, also known as piperazine estrone sulfate and sold under the brand names Harmogen, Improvera, Ogen, Ortho-Est, and Sulestrex among others, is an estrogen medication which is used mainly in menopausal hormone therapy in the treatment of menopausal symptoms. It is a salt of estrone sulfate and piperazine, and is transformed into estrone and estradiol in the body. It is taken by mouth.

Etoperidone, associated with several brand names, is an atypical antidepressant which was developed in the 1970s and either is no longer marketed or was never marketed. It is a phenylpiperazine related to trazodone and nefazodone in chemical structure and is a serotonin antagonist and reuptake inhibitor (SARI) similarly to them.

Cloperastine (INN) or cloperastin, in the forms of cloperastine hydrochloride (JAN) and cloperastine fendizoate, is an antitussive and antihistamine that is marketed as a cough suppressant in Japan, Hong Kong, and in some European countries. It was first introduced in 1972 in Japan, and then in Italy in 1981.

Chlorproethazine, sold under the brand name Neuriplege, is a drug of the phenothiazine group described as a muscle relaxant or tranquilizer which is or has been marketed in Europe as a topical cream for the treatment of muscle pain. It has been associated with photoallergic contact dermatitis.

Oxaflozane (INN) (brand name Conflictan) is an antidepressant and anxiolytic drug that was introduced by Solvay in France in 1982 for the treatment of depression but has since been discontinued. It is a prodrug of flumexadol (N-dealkyloxaflozane; 2-(3-trifluoromethylphenyl)morpholine; CERM-1841 or 1841-CERM), which is reported to act as an agonist of the serotonin 5-HT1A (pKi = 7.1) and 5-HT2C (pKi = 7.5) receptors and, to a much lesser extent, of the 5-HT2A (pKi = 6.0) receptor. In addition to its serotonergic properties, oxaflozane may also produce anticholinergic side effects at high doses, namely in overdose.

Chloral betaine, also known as cloral betaine (INN), is a sedative-hypnotic drug. It was introduced by Mead Johnson in the United States in 1963. It is a betaine complex of trimethylglycine with chloral hydrate, which acts as an extended-acting formulation of chloral hydrate which is then metabolized into trichloroethanol, which is responsible for most or all of its effects.

Carfenazine (INN), or carphenazine (BAN), also known as carphenazine maleate (USAN), is an antipsychotic and tranquilizer of the phenothiazine group that was withdrawn from the market.

Acefluranol, also known as 2,3-bis(3,4-diacetoxy-5-fluorophenyl)pentane, is a nonsteroidal antiestrogen of the stilbestrol group that was never marketed.

Acefurtiamine (INN) is a vitamin B1 analog in a manner similar to the GABAergic activity of the thiamine derivative clomethiazole. It functions as an analgesic agent at sufficient doses.

Cinnamedrine, also known as N-cinnamylephedrine, is a sympathomimetic drug with similar effects relative to those of ephedrine. It also has some local anesthetic activity. Cinnamedrine was previously used, in combination with analgesics, as an antispasmodic to treat dysmenorrhea in the over-the-counter drug Midol in the 1980s. There is a case report of the drug being abused as a psychostimulant.

Penmesterol, or penmestrol, also known as 17α-methyltestosterone 3-cyclopentyl enol ether, is a synthetic, orally active anabolic-androgenic steroid (AAS) that was developed in the early 1960s. It is the 3-cyclopentyl enol ether of methyltestosterone.
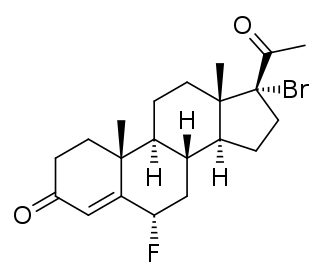
Haloprogesterone, sold under the brand name Prohalone, is a progestin medication which was previously marketed by Ayerst but is now no longer available.

Orestrate, also known as estradiol 3-propionate 17β-(1-cyclohexenyl) ether, is an estrogen medication and estrogen ester which was never marketed. It is the C3 propionate ester and C17β-(1-cyclohexenyl) ether of estradiol.
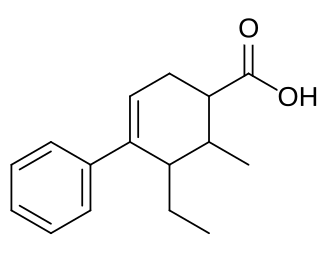
Fenestrel is a synthetic, nonsteroidal estrogen that was developed as a postcoital contraceptive in the 1960s but was never marketed. Synthesized by Ortho Pharmaceutical in 1961 and studied extensively, it was coined the "morning-after-pill" or "postcoital antifertility agent". Fenestrel is a seco analogue of doisynolic acid, and a member of the cyclohexenecarboxylic acid series of estrogens.

Atrimustine, also known as bestrabucil or busramustine, is a cytostatic antineoplastic agent which was under development in Japan by Kureha Chemicals for the treatment of breast cancer and non-Hodgkin's lymphoma as well as for the prevention of graft-versus-host disease in bone marrow transplant recipients. It is the benzoate ester of an ester conjugate of estradiol and chlorambucil, which results in targeted/site-directed cytostatic activity toward estrogen receptor–positive tissues such as breast and bone. It reached preregistration for the treatment of cancer but was ultimately discontinued. Estrogenicic side effects of atrimustine in clinical trials included vaginal bleeding and gynecomastia. The drug was first patented in 1980.

Amadinone (INN), also known as 19-norchlormadinone, is a steroidal progestin of the 19-norprogesterone and 17α-hydroxyprogesterone groups that was synthesized and characterized in 1968 but was never marketed. It has antigonadotropic properties, and for this reason, is a functional antiandrogen. An acetate ester, amadinone acetate, also exists, but similarly was never marketed.

Amadinone acetate, also known as 19-norchlormadinone acetate, is a steroidal progestin of the 19-norprogesterone and 17α-hydroxyprogesterone groups that was never marketed. It is the acetate ester of amadinone, which, similarly, was never marketed.

Droloxifene, also known as 3-hydroxytamoxifen, is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that was developed originally in Germany and later in Japan for the treatment of breast cancer, osteoporosis in men and postmenopausal women, and cardiovascular disorders but was abandoned and never marketed. It reached phase II and phase III clinical trials for these indications before development was discontinued in 2000. The drug was found to be significantly less effective than tamoxifen in the treatment of breast cancer in two phase III clinical trials.

Prednisolamate, also known as prednisolone 21-diethylaminoacetate, is a synthetic corticosteroid. It is or was a component of Etaproctene, which contains lidocaine, prednisolamate hydrochloride, and tetryzoline.

Monalazone, used as monalazone disodium and sold under the brand names Naclobenz-Natrium, Spergisin, and Speton, is a vaginal disinfectant or antiseptic and spermicidal contraceptive. It is a sulfonylbenzoic acid derivative and is closely related structurally to halazone. The compound was synthesized in 1937. A vaginal tablet combination of 0.125 mg estradiol benzoate and 10 mg monalazone was previously marketed under the brand name Malun 25.




















