Cushing's syndrome is a collection of signs and symptoms due to prolonged exposure to glucocorticoids such as cortisol. Signs and symptoms may include high blood pressure, abdominal obesity but with thin arms and legs, reddish stretch marks, a round red face, a fat lump between the shoulders, weak muscles, weak bones, acne, and fragile skin that heals poorly. Women may have more hair and irregular menstruation. Occasionally there may be changes in mood, headaches, and a chronic feeling of tiredness.
Nelson's syndrome is a rare disorder that sometimes occurs in patients in about 15-25% of patients who have had both adrenal glands removed to treat Cushing's disease. In patients with pre-existing adrenocorticotropic hormone (ACTH)-secreting pituitary adenomas, loss of adrenal feedback following bilateral adrenalectomy can trigger the rapid growth of the tumor, leading to visual symptoms and hyperpigmentation. The severity of the disease is dependent upon the effect of ACTH release on the skin, pituitary hormone loss from mass compression, as well as invasion into surrounding structures around the pituitary gland.

Telapristone (INN), as telapristone acetate, is a synthetic, steroidal selective progesterone receptor modulator (SPRM) related to mifepristone which is under development by Repros Therapeutics for the treatment of breast cancer, endometriosis, and uterine fibroids. It was originally developed by the National Institutes of Health (NIH), and, as of 2017, is in phase II clinical trials for the aforementioned indications. In addition to its activity as an SPRM, the drug also has some antiglucocorticoid activity.

Bupropion/zonisamide is an experimental combination of bupropion which was under development for the treatment of obesity. Bupropion is a norepinephrine–dopamine reuptake inhibitor and nicotinic acetylcholine receptor antagonist, while zonisamide is an anticonvulsant acting as a sodium channel blocker, T-type calcium channel blocker, and weak carbonic anhydrase inhibitor. The combination was being developed by Orexigen Therapeutics and reached phase II clinical trials prior to discontinuation.

Intepirdine (INN; developmental codes SB-742457, RVT-101) is a selective 5-HT6 receptor antagonist with potential cognition, memory, and learning-enhancing effects. It was under development by GlaxoSmithKline for the treatment of Alzheimer's disease and demonstrated some preliminary efficacy in phase II clinical trials. GSK chose not to continue development and sold the rights to Axovant Sciences for $5 million in December 2014.

Samidorphan, is an opioid antagonist which in the form of olanzapine/samidorphan is used in the treatment of schizophrenia and bipolar disorder. Samidorphan reduces the weight gain associated with olanzapine. Samidorphan is taken by mouth.

AZD-5423 is a nonsteroidal glucocorticoid and phase II experimental drug being developed by AstraZeneca and disclosed at the spring 2013 American Chemical Society meeting in New Orleans to treat respiratory diseases and in particular chronic obstructive pulmonary disease.
Corcept Therapeutics Inc. is a pharmaceutical company engaged in the discovery, development and commercialization of drugs for the treatment of severe metabolic, psychiatric and oncologic disorders. Corcept has focused on the adverse effects of excess cortisol, studying new compounds that may mitigate those effects. Its executive team is headed by CEO, president and director Joseph K. Belanoff, MD, and by chief medical officer Robert S. Fishman, MD.

BTRX-246040, also known as LY-2940094, is a potent and selective nociceptin receptor antagonist which is under development by BlackThorn Therapeutics and Eli Lilly for the treatment of major depressive disorder (MDD). It has demonstrated proof-of-concept clinical efficacy for depression. As of 2017, it is in phase II clinical trials for the treatment of MDD. It was also under investigation for the treatment of alcoholism, and similarly reached phase II clinical studies for this indication, but development was discontinued.
Depatuxizumab mafodotin is an antibody-drug conjugate designed for the treatment of cancer. It is composed of an EGFR IGg1 monoclonal antibody (depatuxizumab) conjugated to the tubulin inhibitor monomethyl auristatin F via a stable maleimidocaproyl link.

Droloxifene, also known as 3-hydroxytamoxifen, is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that was developed originally in Germany and later in Japan for the treatment of breast cancer, osteoporosis in men and postmenopausal women, and cardiovascular disorders but was abandoned and never marketed. It reached phase II and phase III clinical trials for these indications before development was discontinued in 2000. The drug was found to be significantly less effective than tamoxifen in the treatment of breast cancer in two phase III clinical trials.

Levoketoconazole, also known as (2S,4R)-ketoconazole, is a steroidogenesis inhibitor that is under development by Strongbridge Biopharma for the treatment of Cushing's syndrome. It is currently in phase III clinical trials for this indication. The drug is the levorotatory or (2S,4R) enantiomer of ketoconazole. It is expected to have greater potency, efficacy, and safety, including a lower risk of hepatotoxicity, relative to racemic ketoconazole.

Larotrectinib, sold under the brand name Vitrakvi, is a medication for the treatment of cancer. It is an inhibitor of tropomyosin kinase receptors TrkA, TrkB, and TrkC. It was discovered by Array BioPharma and licensed to Loxo Oncology in 2013.

Pavinetant (INN, USAN; developmental code names MLE-4901, AZD-4901, AZ-12472520, AZD-2624), is a small-molecule, orally active, selective neurokinin-3 (NK3) receptor antagonist which was under development by AstraZeneca and Millendo Therapeutics for the treatment of hot flashes and polycystic ovary syndrome (PCOS). It was also under investigation for the treatment of schizophrenia, but development was discontinued for this indication due to lack of effectiveness. In November 2017, development of the medication for hot flashes and PCOS was also terminated after its developer assessed the clinical risks and benefits.
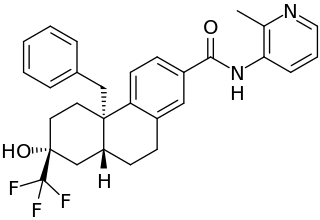
Dagrocorat is a nonsteroidal but steroid-like selective glucocorticoid receptor modulator (SGRM) which was under development for the treatment of rheumatoid arthritis but was never marketed. It is described as a partial agonist and "dissociable" agonist of the glucocorticoid receptor. The drug reached phase I clinical trials prior to the discontinuation of its development. The C2α dihydrogen phosphate ester of dagrocorat, fosdagrocorat, was also under investigation, but its development was terminated as well.
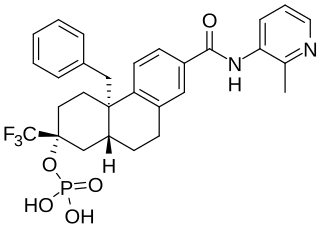
Fosdagrocorat is a nonsteroidal but steroid-like selective glucocorticoid receptor modulator (SGRM) which was under development for the treatment of rheumatoid arthritis but was never marketed. It is the C2 dihydrogen phosphate ester of dagrocorat, and acts as a prodrug of dagrocorat with improved pharmacokinetics. The drug reached phase II clinical trials prior to the discontinuation of its development.

Foliglurax (developmental code names PXT-002331, DT2331) is a positive allosteric modulator of the metabotropic glutamate receptor 4 (mGluR4) which is under development by Prexton Therapeutics for the treatment of Parkinson's disease. As of July 2017, it is in phase II clinical trials.
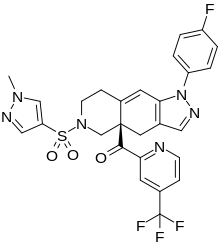
IONIS-GCCRRx, also known as ISIS-426115, is an antiglucocorticoid which is under development by Ionis Pharmaceuticals for the treatment of diabetes mellitus type 2. It has also been under investigation for the treatment of Cushing's syndrome, but no development has been reported. The drug is an antisense oligonucleotide against the glucocorticoid receptor. As of December 2017, it is in phase II clinical trials for diabetes mellitus type 2.
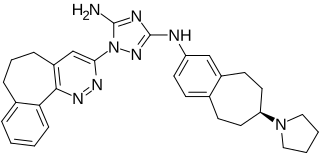
Bemcentinib, also known as BGB324 or R428, is an experimental oral small molecule that is an inhibitor of AXL kinase. Bemcentinib was licensed from Rigel Pharmaceuticals by BerGenBio and currently undergoing six Phase II trials in various solid and hematological tumors as monotherapy and in combination with immunotherapy, chemotherapy, and targeted therapeutics.
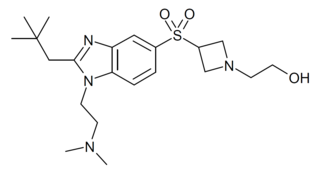
RQ-00202730 is a benzimidazole derived drug that acts as a potent and highly selective agonist for the CB2 cannabinoid receptor, with a Ki value of 19nM at CB2 and more than 4000x selectivity over CB1, though it also shows some activity as an antagonist of the unrelated 5-HT2B serotonin receptor. It has analgesic and antiinflammatory effects in animal studies, and was developed for the treatment of irritable bowel syndrome, but was ultimately discontinued from development following disappointing results in Phase II clinical trials.