
Prednisolone is a steroid medication used to treat certain types of allergies, inflammatory conditions, autoimmune disorders, and cancers. Some of these conditions include adrenocortical insufficiency, high blood calcium, rheumatoid arthritis, dermatitis, eye inflammation, asthma, and multiple sclerosis. It can be taken by mouth, injected into a vein, used topically as a skin cream, or as eye drops.

Triamcinolone acetonide is a synthetic corticosteroid medication used topically to treat various skin conditions, to relieve the discomfort of mouth sores, and intra-articularly by proceduralists to treat various joint conditions. It is also injected intralesionally to treat inflammation in some parts of the body, particularly the skin. In nasal spray form, it is used to treat allergic rhinitis. It is a more potent derivative of triamcinolone, and is about eight times as potent as prednisone.
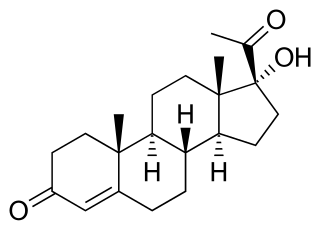
17α-Hydroxyprogesterone (17α-OHP), also known as 17-OH progesterone (17-OHP), or hydroxyprogesterone (OHP), is an endogenous progestogen steroid hormone related to progesterone. It is also a chemical intermediate in the biosynthesis of many other endogenous steroids, including androgens, estrogens, glucocorticoids, and mineralocorticoids, as well as neurosteroids.

Fluorometholone, also known as 6α-methyl-9α-fluoro-11β,17α-dihydroxypregna-1,4-diene-3,20-dione, is a synthetic glucocorticoid which is used in the treatment of inflammatory eye diseases. The C17α acetate ester, fluorometholone acetate, is also a glucocorticoid and is used for similar indications.

Fluorometholone acetate, also known as oxylone acetate and sold under the brand names Flarex, Florate, and Omnitrol, is a synthetic glucocorticoid corticosteroid and a corticosteroid ester, as well as a progestogen and progestogen ester. It is the C17α acetate ester of fluorometholone.

Amcinonide is a topical glucocorticoid used to treat itching, redness and swelling associated with several dermatologic conditions such as atopic dermatitis and allergic contact dermatitis. Amcinonide can also be classified as a multi-functional small molecule corticosteroid, which has been approved by the FDA and is currently marketed as an ointment, lotion, or cream. It acts as both a transcription factor for responses to glucocorticoids and modulator for other transcription factors while also regulating phospholipase A2 activity.

Chlormadinone acetate (CMA), sold under the brand names Belara, Gynorelle, Lutéran, and Prostal among others, is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy, as a component of menopausal hormone therapy, in the treatment of gynecological disorders, and in the treatment of androgen-dependent conditions like enlarged prostate and prostate cancer in men and acne and hirsutism in women. It is available both at a low dose in combination with an estrogen in birth control pills and, in a few countries like France and Japan, at low, moderate, and high doses alone for various indications. It is taken by mouth.

Medrogestone, sold under the brand name Colprone among others, is a progestin medication which has been used in menopausal hormone therapy and in the treatment of gynecological disorders. It is available both alone and in combination with an estrogen. It is taken by mouth.

Cyproterone, also known by its developmental code name SH-80881, is a steroidal antiandrogen which was studied in the 1960s and 1970s but was never introduced for medical use. It is an analogue of cyproterone acetate (CPA), an antiandrogen, progestin, and antigonadotropin which was introduced instead of cyproterone and is widely used as a medication. Cyproterone and CPA were among the first antiandrogens to be developed.

Segesterone acetate (SGA), sold under the brand names Nestorone, Elcometrine, and Annovera, is a progestin medication which is used in birth control and in the treatment of endometriosis in the United States, Brazil, and other South American countries. It is available both alone and in combination with an estrogen. It is not effective by mouth and must be given by other routes, most typically as a vaginal ring or implant that is placed into fat.

Seviteronel is an experimental cancer medication which is under development by Viamet Pharmaceuticals and Innocrin Pharmaceuticals for the treatment of prostate cancer and breast cancer. It is a nonsteroidal CYP17A1 inhibitor and works by inhibiting the production of androgens and estrogens in the body. As of July 2017, seviteronel is in phase II clinical trials for both prostate cancer and breast cancer. In January 2016, it was designated fast-track status by the United States Food and Drug Administration for prostate cancer. In April 2017, seviteronel received fast-track designation for breast cancer as well.

Clomegestone acetate (USAN), or clomagestone acetate, also known as 6-chloro-17α-acetoxy-16α-methylpregna-4,6-diene-3,20-dione, is a steroidal progestin of the 17α-hydroxyprogesterone group which was developed as an oral contraceptive but was never marketed. It is the acetate ester of clomegestone, which, similarly to clomegestone acetate, was never marketed. Clomegestone acetate is also the 17-desoxy cogener of clometherone, and is somewhat more potent in comparison. Similarly to cyproterone acetate, clomegestone acetate has been found to alter insulin receptor concentrations in adipose tissue, and this may indicate the presence of glucocorticoid activity.

A steroidal antiandrogen (SAA) is an antiandrogen with a steroidal chemical structure. They are typically antagonists of the androgen receptor (AR) and act both by blocking the effects of androgens like testosterone and dihydrotestosterone (DHT) and by suppressing gonadal androgen production. SAAs lower concentrations of testosterone through simulation of the negative feedback inhibition of the hypothalamus. SAAs are used in the treatment of androgen-dependent conditions in men and women, and are also used in veterinary medicine for the same purpose. They are the converse of nonsteroidal antiandrogens (NSAAs), which are antiandrogens that are not steroids and are structurally unrelated to testosterone.

Flumedroxone acetate, sold under the brand names Demigran and Leomigran, is a progestin medication which is or has been used as an antimigraine agent. It is taken by mouth.

Pregnenolone acetate, also known as pregn-5-en-3β-ol-20-one 3β-acetate, is a synthetic pregnane steroid and an ester of pregnenolone which is described as a glucocorticoid and as a skin-conditioning and skin anti-aging agent. It has been reported to reduce wrinkles in elderly women when applied in the form of a 0.5% topical cream, effects which were suggested to be due to improved hydration of the skin. Pregnenolone acetate has been marketed in France in a topical cream containing 1% pregnenolone acetate and 10% "sex hormone" for the treatment of premature skin aging but was withdrawn from the market in 1992. Although the medication has been described by some sources as a glucocorticoid, other authors have stated that systemic pregnenolone acetate has no undesirable metabolic or toxic effects even at high doses.
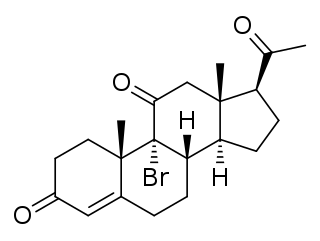
Bromoketoprogesterone (BKP), also known as 9α-bromo-11-oxoprogesterone (BOP), and known by the tentative brand name Braxarone (Squibb), is an orally active progestin which does not appear to have been marketed.

Isoflupredone, also known as deltafludrocortisone and 9α-fluoroprednisolone, is a synthetic glucocorticoid corticosteroid which was never marketed.

Fluoromedroxyprogesterone acetate is a synthetic steroid medication which was under development by Meiji Dairies Corporation in the 1990s and 2000s for the potential treatment of cancers but was never marketed. It is described as an antiangiogenic agent, with about two orders of magnitude greater potency for inhibition of angiogenesis than its parent compound medroxyprogesterone acetate. FMPA showed about the same affinities for the progesterone and glucocorticoid receptors as MPA. It reached the preclinical phase of research prior to the discontinuation of its development.


















