
Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids, are involved in a wide range of physiological processes, including stress response, immune response, and regulation of inflammation, carbohydrate metabolism, protein catabolism, blood electrolyte levels, and behavior.

A steroid hormone is a steroid that acts as a hormone. Steroid hormones can be grouped into two classes: corticosteroids and sex steroids. Within those two classes are five types according to the receptors to which they bind: glucocorticoids and mineralocorticoids and androgens, estrogens, and progestogens. Vitamin D derivatives are a sixth closely related hormone system with homologous receptors. They have some of the characteristics of true steroids as receptor ligands.

Prednisone is a glucocorticoid medication mostly used to suppress the immune system and decrease inflammation in conditions such as asthma, COPD, and rheumatologic diseases. It is also used to treat high blood calcium due to cancer and adrenal insufficiency along with other steroids. It is taken by mouth.

Glucocorticoids are a class of corticosteroids, which are a class of steroid hormones. Glucocorticoids are corticosteroids that bind to the glucocorticoid receptor that is present in almost every vertebrate animal cell. The name "glucocorticoid" is a portmanteau and is composed from its role in regulation of glucose metabolism, synthesis in the adrenal cortex, and its steroidal structure. A less common synonym is glucocorticosteroid.

11β-Hydroxysteroid dehydrogenase (HSD-11β or 11β-HSD) is a family of enzymes that catalyze the conversion of inert 11 keto-products (cortisone) to active cortisol, or vice versa, thus regulating the access of glucocorticoids to the steroid receptors:

Fluorometholone acetate, also known as oxylone acetate and sold under the brand names Flarex, Florate, and Omnitrol, is a synthetic glucocorticoid corticosteroid and a corticosteroid ester, as well as a progestogen and progestogen ester. It is the C17α acetate ester of fluorometholone.
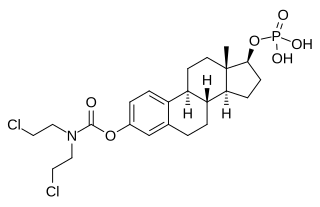
Estramustine phosphate (EMP), also known as estradiol normustine phosphate and sold under the brand names Emcyt and Estracyt, is a dual estrogen and chemotherapy medication which is used in the treatment of prostate cancer in men. It is taken multiple times a day by mouth or by injection into a vein.
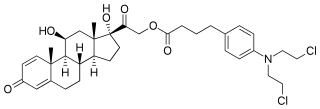
Prednimustine, sold under the brand names Mostarina and Sterecyst, is a medication which is used in chemotherapy in the treatment of leukemias and lymphomas. It is the ester formed from two other drugs, prednisolone and chlorambucil. Rarely, it has been associated with myoclonus.
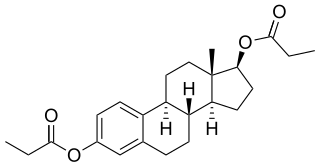
Estradiol dipropionate (EDP), sold under the brand names Agofollin, Di-Ovocylin, and Progynon DP among others, is an estrogen medication which has been used in hormone therapy for menopausal symptoms and low estrogen levels in women and in the treatment of gynecological disorders. It has also been used in feminizing hormone therapy for transgender women and in the treatment of prostate cancer in men. Although widely used in the past, estradiol dipropionate has largely been discontinued and is mostly no longer available today. It appears to remain in use only in Japan, Macedonia, and Australia. Estradiol dipropionate is given by injection into muscle at intervals ranging from once or twice a week to once every week and a half to two weeks.

Atrimustine (INN), also known as bestrabucil or busramustine, is a cytostatic antineoplastic agent which was under development in Japan by Kureha Chemicals for the treatment of breast cancer and non-Hodgkin's lymphoma as well as for the prevention of graft-versus-host disease in bone marrow transplant recipients. It is the benzoate ester of an ester conjugate of estradiol and chlorambucil, which results in targeted/site-directed cytostatic activity toward estrogen receptor-positive tissues such as breast and bone. It reached preregistration for the treatment of cancer but was ultimately discontinued. Estrogenicic side effects of atrimustine in clinical trials included vaginal bleeding and gynecomastia. The drug was first patented in 1980.

Quingestrone, also known as progesterone 3-cyclopentyl enol ether (PCPE) and sold under the brand name Enol-Luteovis, is a progestin medication which was previously used in birth control pills in Italy but is now no longer marketed. It is taken by mouth.
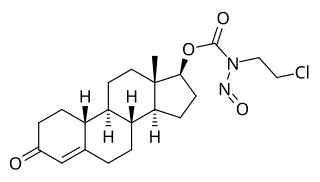
LS-1727 is a synthetic, injected anabolic–androgenic steroid (AAS) and a nitrosocarbamate ester of nandrolone (19-nortestosterone) which was developed as a cytostatic antineoplastic agent but was never marketed.
Phenestrol, or fenestrol, also known as hexestrol bis[4-[bis(2-chloroethyl)amino]phenylacetate, is a synthetic, nonsteroidal estrogen and cytostatic antineoplastic agent and a chlorphenacyl nitrogen mustard ester of hexestrol which was developed in the early 1960s for the treatment of hormone-dependent tumors but was never marketed.

Estradiol mustard, also known as chlorphenacyl estradiol diester, as well as estradiol 3,17β-bis(4- phenyl)acetate, is a synthetic, steroidal estrogen and cytostatic antineoplastic agent and a chlorphenacyl nitrogen mustard-coupled estrogen ester that was never marketed. It is selectively distributed into estrogen receptor (ER)-positive tissues such as ER-expressing tumors like those seen in breast and prostate cancers. For this reason, estradiol mustard and other cytostatic-linked estrogens like estramustine phosphate have reduced toxicity relative to non-linked nitrogen mustard cytostatic antineoplastic agents. However, they may stimulate breast tumor growth due to their inherent estrogenic activity and are said to be devoid of major therapeutic efficacy in breast cancer, although estramustine phosphate has been approved for and is used in the treatment of prostate cancer.
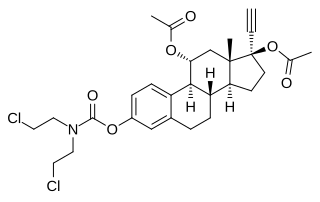
Cytestrol acetate is a steroidal antiestrogen and a cytostatic antineoplastic agent which was developed for the treatment of breast cancer but was never marketed. It is an 11α-hydroxylated derivative of ethinylestradiol in which a bis(2-chloroethyl)amine nitrogen mustard moiety has been attached as an ester at the C3 position and acetate esters have been attached at the C11α and C17β positions. The mechanism of action of cytestrol acetate in breast cancer is two-fold: (1) acting as an antiestrogen similarly to fulvestrant or ICI-164384; and (2) having cytostatic actions via the carbamate–nitrogen mustard moiety analogously to estramustine phosphate. The drug shows potent efficacy against breast cancer superior to that of tamoxifen in in vitro models.

Testifenon, also known as testiphenon, testiphenone, chlorphenacyl dihydrotestosterone ester, or dihydrotestosterone 17β-(4- phenyl)acetate, is a synthetic anabolic–androgenic steroid (AAS) and a cytostatic antineoplastic agent that was never marketed. It is an androgen ester – specifically, a chlorphenacyl nitrogen mustard ester of dihydrotestosterone (DHT) – and acts as a prodrug of these two components in the body. The drug was developed in Russia as a tissue-selective cytostatic drug for the treatment of various cancers occurring in androgen receptor-expressing tissues that would have reduced side effects and toxicity relative to other chemotherapy drugs.

Sturamustine, also known as dehydroepiandrosterone (DHEA) 17β-N-(2-chloroethyl)-N-nitrosourea, is a synthetic androstane steroid and a C17β nitrosourea conjugate of dehydroepiandrosterone (DHEA) which was developed as a cytostatic antineoplastic agent for the treatment of hormone-dependent tumors but was never marketed. It was synthesized in 1982.

Cymegesolate, also known as cypionyl megestrol acetate or as megestrol acetate 3β-cypionate, is a progestin medication which was never marketed. It was developed in China in the late 1970s and early to mid 1980s for use as a hormonal contraceptive. The medication was formulated at a dose of 50–100 mg in combination with a "trace" dose of 0.25–0.5 mg quinestrol as a long-lasting, once-a-month combined oral contraceptive pill. This combination has been studied in 1,213 women across a total of 9,651 menstrual cycles, with contraceptive effectiveness of over 99.13% and "very few side effects." At the high dose, it showed an anovulation rate of only about 60%, and instead mediated its contraceptive effects via a marked anti-implantation effect.

5α-Dihydroethisterone is an active metabolite of the formerly clinically used but now-discontinued progestin ethisterone and the experimental and never-marketed hormonal antineoplastic agent ethynylandrostanediol (HE-3235). Its formation from its parent drugs is catalyzed by 5α-reductase in tissues that express the enzyme in high amounts like the liver, skin, hair follicles, and prostate gland. 5α-DHET has significant affinity for steroid hormone receptors and may contribute importantly to the activities of its parent drugs.


















