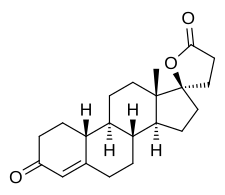
Spironolactone, sold under the brand name Aldactone among others, is a medication that is primarily used to treat fluid build-up due to heart failure, liver scarring, or kidney disease. It is also used in the treatment of high blood pressure, low blood potassium that does not improve with supplementation, early puberty in boys, acne and excessive hair growth in women, and as a part of feminizing hormone therapy in trans women. Spironolactone is taken by mouth.
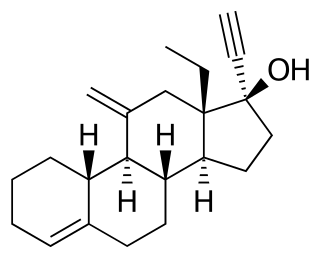
Desogestrel is a progestin medication which is used in birth control pills for women. It is also used in the treatment of menopausal symptoms in women. The medication is available and used alone or in combination with an estrogen. It is taken by mouth.

A mineralocorticoid receptor antagonist or aldosterone antagonist, is a diuretic drug which antagonizes the action of aldosterone at mineralocorticoid receptors. This group of drugs is often used as adjunctive therapy, in combination with other drugs, for the management of chronic heart failure. Spironolactone, the first member of the class, is also used in the management of hyperaldosteronism and female hirsutism. Most antimineralocorticoids, including spironolactone, are steroidal spirolactones. Finerenone is a nonsteroidal antimineralocorticoid.

Ethisterone, also known as ethinyltestosterone, pregneninolone, and anhydrohydroxyprogesterone and formerly sold under the brand names Proluton C and Pranone among others, is a progestin medication which was used in the treatment of gynecological disorders but is now no longer available. It was used alone and was not formulated in combination with an estrogen. The medication is taken by mouth.

Dimethisterone, formerly sold under the brand names Lutagan and Secrosteron among others, is a progestin medication which was used in birth control pills and in the treatment of gynecological disorders but is now no longer available. It was used both alone and in combination with an estrogen. It is taken by mouth.
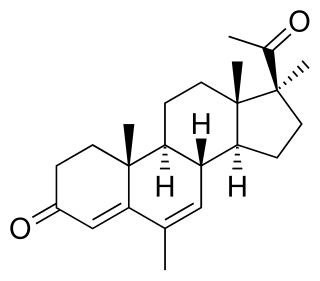
Medrogestone, sold under the brand name Colprone among others, is a progestin medication which has been used in menopausal hormone therapy and in the treatment of gynecological disorders. It is available both alone and in combination with an estrogen. It is taken by mouth.

Altrenogest, sold under the brand names Swinemate and Altren manufactured by Aurora Pharmaceutical and Regumate manufactured by Merck, is a progestin of the 19-nortestosterone group which is widely used in veterinary medicine to suppress or synchronize estrus in horses and pigs. It is available for veterinary use in both Europe and the United States.

Spirolactones are a class of functional group in organic chemistry featuring a cyclic ester attached spiro to another ring system. The name is also used to refer to a class of synthetic steroids, called steroid-17α-spirolactones, 17α-spirolactosteroids, or simply 17α-spirolactones, which feature their spirolactone group at the C17α position. They are antimineralocorticoids, or antagonists of the mineralocorticoid receptor, and have been employed clinically as potassium-sparing diuretics. Some also possess progestogenic and/or antiandrogen properties, which have both contributed to side effects and been utilized for medical indications. The spirolactones were developed by G. D. Searle & Company in the 1950s and thereafter and were denoted as "SC" compounds.

Cyproterone, also known by its developmental code name SH-80881, is a steroidal antiandrogen which was studied in the 1960s and 1970s but was never introduced for medical use. It is an analogue of cyproterone acetate (CPA), an antiandrogen, progestin, and antigonadotropin which was introduced instead of cyproterone and is widely used as a medication. Cyproterone and CPA were among the first antiandrogens to be developed.

Segesterone acetate (SGA), sold under the brand names Nestorone, Elcometrine, and Annovera, is a progestin medication which is used in birth control and in the treatment of endometriosis in the United States, Brazil, and other South American countries. It is available both alone and in combination with an estrogen. It is not effective by mouth and must be given by other routes, most typically as a vaginal ring or implant that is placed into fat.
19-Norprogesterone, also known as 19-norpregn-4-ene-3,20-dione, is a steroidal progestin and close analogue of the sex hormone progesterone, lacking only the C19 methyl group of that molecule. It was first synthesized in 1944 in the form of a mixture that also included unnatural stereoisomers of progesterone, and this mixture was found to be at least equivalent to progesterone in terms of progestogenic activity. Subsequent investigations revealed that 17-isoprogesterone and 14-iso-17-isoprogesterone are devoid of progestogenic activity. 19-Norprogesterone was resynthesized in 1951 with an improved method, and was confirmed to be the component of the mixture synthesized in 1944 that was responsible for its progestogenic activity. In 1953, a paper was published showing that 19-norprogesterone possessed 4- to 8-fold the activity of progesterone in the Clauberg assay in rabbits, and at the time of this discovery, 19-norprogesterone was the most potent progestogen known.

Benorterone, also known by its developmental code name SKF-7690 and as 17α-methyl-B-nortestosterone, is a steroidal antiandrogen which was studied for potential medical use but was never marketed. It was the first known antiandrogen to be studied in humans. It is taken by mouth or by application to skin.

Prorenone is a steroidal antimineralocorticoid of the spirolactone group related to spironolactone that was never marketed. It is the lactonic form of prorenoic acid (prorenoate), and prorenoate potassium (SC-23992), the potassium salt of prorenoic acid, also exists. Prorenoate potassium is about 8 times more potent than spironolactone as an antimineralocorticoid in animals, and it may act as a prodrug to prorenone. In addition to the mineralocorticoid receptor, prorenone also binds to the glucocorticoid, androgen, and progesterone receptors. The antiandrogenic potency of prorenone in vivo in animals is close to that of spironolactone. Similarly to spironolactone, prorenone is also a potent inhibitor of aldosterone biosynthesis.

Mexrenone is a steroidal antimineralocorticoid of the spirolactone group related to spironolactone that was never marketed. It is the lactonic form of mexrenoic acid (mexrenoate), and mexrenoate potassium (SC-26714), the potassium salt of mexrenoic acid, also exists. In addition to the mineralocorticoid receptor, mexrenone also binds to the glucocorticoid, androgen, and progesterone receptors. Relative to spironolactone, it has markedly reduced antiandrogen activity. Eplerenone is the 9-11α-epoxy analogue of mexrenone.

SC-5233, also known as 6,7-dihydrocanrenone or 20-spirox-4-ene-3,20-dione, is a synthetic, steroidal antimineralocorticoid of the spirolactone group which was developed by G. D. Searle & Company in the 1950s but was never marketed. It was the first synthetic antagonist of the mineralocorticoid receptor to have been identified and tested in humans. The drug was found to lack appreciable oral bioavailability and to be of low potency when administered parenterally, but it nonetheless produced a mild diuretic effect in patients with congestive heart failure. SC-8109, the 19-nor (19-demethyl) analogue, was developed and found to have improved oral bioavailability and potency, but still had low potency. Spironolactone followed and had both good oral bioavailability and potency, and was the first synthetic antimineralocorticoid to be marketed. It has about 46-fold higher oral potency than SC-5233.

Spirorenone (INN) is a steroidal antimineralocorticoid of the spirolactone group that was never marketed. Spirorenone possesses 5–8 times the antimineralocorticoid activity of spironolactone in animal studies. The initial discovery of spirorenone was deemed a great success, as no compound with greater antimineralocorticoid activity had been developed since spironolactone in 1957. Moreover, spirorenone itself has virtually no affinity for the androgen receptor while its progestogenic activity shows species differences, being somewhat greater than that of spironolactone in rabbits but absent in mice and rats. As such, it was characterized as a highly potent antimineralocorticoid with far fewer hormonal side effects relative to spironolactone.

A steroidal antiandrogen (SAA) is an antiandrogen with a steroidal chemical structure. They are typically antagonists of the androgen receptor (AR) and act both by blocking the effects of androgens like testosterone and dihydrotestosterone (DHT) and by suppressing gonadal androgen production. SAAs lower concentrations of testosterone through simulation of the negative feedback inhibition of the hypothalamus. SAAs are used in the treatment of androgen-dependent conditions in men and women, and are also used in veterinary medicine for the same purpose. They are the converse of nonsteroidal antiandrogens (NSAAs), which are antiandrogens that are not steroids and are structurally unrelated to testosterone.

Dicirenone is a synthetic, steroidal antimineralocorticoid of the spirolactone group which was developed as a diuretic and antihypertensive agent but was never marketed. It was synthesized and assayed in 1974. Similarly to other spirolactones like spironolactone, dicirenone also possesses antiandrogen activity, albeit with relatively reduced affinity.

7α-Thioprogesterone is a synthetic, steroidal, and potent antimineralocorticoid (putative) and antiandrogen which was developed by G. D. Searle & Co and was described in the late 1970s and early 1980s but was never developed or introduced for medical use. It is a derivative of progesterone (pregn-4-ene-3,20-dione) with a thio (sulfur) substitution at the C7α position, and is related to the spirolactone group of drugs but lacks a γ-lactone ring.

DU-41164, also known as 1,2β-methylene-6-fluoro-17α-acetoxy-δ6-retroprogesterone, is a progestin which was developed by Philips-Duphar in the 1970s and was never marketed. It is a combined derivative of 17α-hydroxyprogesterone and retroprogesterone. The drug shows extremely high potency as a progestogen in animals; it was reported to possess 500 times the affinity of progesterone for the progesterone receptor expressed in rabbit uterus, and showed 600 times the progestogenic potency of subcutaneous progesterone when given orally in animals. The affinity of DU-41164 for the progesterone receptor was described in 1974 as "probably the highest reported for any steroid-receptor interaction". The drug showed no androgenic, anabolic, antiandrogenic, estrogenic, or corticosteroid activity in animals. Although highly potent in animals, DU-41164 produced little or no progestogenic effect at dosages of 50 and 200 µg/day in women, suggesting major species differences. A closely related compound, DU-41165, has been developed as a photoaffinity label for the progesterone receptor.