Acokanthera is a genus of flowering plants in the family Apocynaceae. It comprises 5 species and is generally restricted to Africa, although Acokanthera schimperi also occurs in Yemen. Its sap contains the deadly cardiotoxic glycoside ouabain. The sap is among the most commonly used in arrow poisons, including those used for poaching elephant.

Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids.

Plant Resources of Tropical Africa, known by its acronym PROTA, is a retired NGO and interdisciplinary documentation programme active between 2000 and 2013. PROTA produced a large database and various publications about Africa's useful plants.
Pierreodendron kerstingii is a species of tree in the family Simaroubaceae. It is endemic to West Africa and found in Ivory Coast, Ghana, Togo, and Benin. It is sometimes considered synonym of Pierreodendron africanum, which would then be a widespread species distributed south to Angola and east to the Democratic Republic of the Congo.

Tabernaemontana corymbosa is a species of plant in the family Apocynaceae. It is native to Brunei, China, Indonesia, Laos, Malaysia, Myanmar, Singapore, Thailand, and Vietnam. Glossy green leaves and faintly sweet scented flower. Flowers continuously all year. Frost tolerant. Grows to about 2 metres. Likes full sun to part shade. A number of cultivars are available.

Adenium boehmianum, the Bushman poison, is a poisonous succulent endemic to the mostly dry regions of northern Namibia and southern Angola. The San people boil the root sap and latex to prepare arrow poison, which is sufficient for hunting large mammals, as it contains strong cardiotoxic effects. The leaves, borne only for three months a year, are arranged spirally and are clustered near the branch tips. A plant will flower for only a few weeks in winter. The oblong fruit releases many seeds through a longitudinal slit, which due to their lateral tufts, can be dispersed by wind.

Acalypha ciliata is a species in the botanical family Euphorbiaceae. It occurs widely in Africa where it is eaten as a vegetable, or fed to animals. In West Africa and East Africa it is used as a medicinal plant.

Acalypha fruticosa is a species of flowering plant in the botanical family Euphorbiaceae. It occurs widely in East and southern Africa where it is eaten as a vegetable. It is also an important browse plant for sheep. In East Africa and southern Africa it is used as a medicinal plant. In northern Kenya arrow shafts and beehive lids are made from the stem. From the dried leaves a tea is made in Ethiopia.
Acalypha integrifolia is a species of flowering plant in the botanical family Euphorbiaceae. It is locally used as a medicinal plant. Leaf decoctions are drunk to treat intestinal worms.
Acalypha ornata is a species in the botanical family Euphorbiaceae. In Africa it is widely used as a medicinal plant. The stems are used as fibres for weaving baskets. The leaves are eaten as a vegetable; the plants are also fed to domestic animals. Acalypha ornata is sometimes planted as an ornamental plant.
Acalypha psilostachya is a species in the botanical family Euphorbiaceae. In East Africa it is used as a medicinal plant.

Acalypha villicaulis is a species in the botanical family Euphorbiaceae. In tropical Africa it is widely used as a medicinal plant.

Hymenocardia acida is a plant of the family Phyllanthaceae native to tropical Africa. It is a small tree that grows to 10 m tall. Occurs in the Guinea and Sudanian savannah zones and deciduous woodland, from Senegal eastwards to Ethiopia and southwards reaching Zimbabwe.

Tabernaemontana elegans, the toad tree, is a shrub or small tree in the family Apocynaceae. It is native to eastern Africa.

Stemmadenine is a terpene indole alkaloid. Stemmadenine is believed to be formed from preakuammicine by a carbon-carbon bond cleavage. Cleavage of a second carbon-carbon bond is thought to form dehydrosecodine. The enzymes forming stemmadenine and using it as a substrate remain unknown to date. It is thought to be intermediate compound in many different biosynthetic pathways such as in Aspidosperma species. Many alkaloids are proposed to be produced through intermediate stemmadenine. Some of them are:

Phyllanthus maderaspatensis is a perennial herbaceous species of plant in the family Phyllanthaceae, widespread in tropical and subtropical areas of the old world.

Tabernaemontanine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Tabernaemontana divaricata.

Vobasine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Tabernaemontana divaricata.
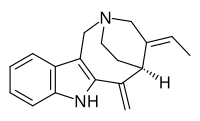
Vinervine is a monoterpene indole alkaloid of the Vinca sub-group. It is a derivative of akuammicine, with one additional hydroxy (OH) group in the indole portion, hence it is also known as 12-hydroxyakuammicine.

Conopharyngine is the major alkaloid present in the leaves and stem-bark of Tabernaemontana pachysiphon and Conopharyngia durissima. It is closely related voacangine and coronaridine. Conopharyngine pseudoindoxyl, a derivative of it, is also found in the same plant Tabernaemontana pachysiphon.