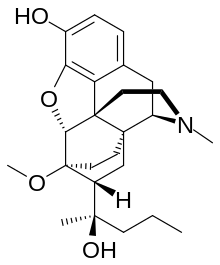
Carfentanil or carfentanyl, sold under the brand name Wildnil, is an extremely potent opioid analgesic used in veterinary medicine to anesthetize large animals such as elephants and rhinoceroses. It is typically administered in this context by tranquilizer dart. Carfentanil has also been used in humans to image opioid receptors. It has additionally been used as a recreational drug, typically by injection, insufflation, or inhalation. Deaths have been reported in association with carfentanil.

Etorphine (M99) is a semi-synthetic opioid possessing an analgesic potency approximately 10,000–30,000 times that of morphine. It was first prepared in 1960 from oripavine, which does not generally occur in opium poppy extract but rather the related plants Papaver orientale and Papaver bracteatum. It was later reproduced in 1963 by a research group at MacFarlan Smith in Gorgie, Edinburgh, led by Kenneth Bentley. It can also be produced from thebaine.

Nalbuphine, sold under the brand names Nubain among others, is an opioid analgesic which is used in the treatment of pain. It is given by injection into a vein, muscle, or fat.

Butorphanol is a morphinan-type synthetic agonist–antagonist opioid analgesic developed by Bristol-Myers. Butorphanol is most closely structurally related to levorphanol. Butorphanol is available as the tartrate salt in injectable, tablet, and intranasal spray formulations. The tablet form is only used in dogs, cats and horses due to low bioavailability in humans.

Piritramide(R-3365, trade names Dipidolor, Piridolan, Pirium and others) is a synthetic opioid analgesic that is marketed in certain European countries including: Austria, Belgium, Czech Republic, Slovenia, Germany and the Netherlands. It comes in free form, is about 0.75x times as potent as morphine and is given parenterally for the treatment of severe pain. Nausea, vomiting, respiratory depression and constipation are believed to be less frequent with piritramide than with morphine, and it produces more rapid-onset analgesia when compared to morphine and pethidine. After intravenous administration the onset of analgesia is as little as 1–2 minutes, which may be related to its great lipophilicity. The analgesic and sedative effects of piritramide are believed to be potentiated with phenothiazines and its emetic (nausea/vomiting-inducing) effects are suppressed. The volume of distribution is 0.7-1 L/kg after a single dose, 4.7-6 L/kg after steady-state concentrations are achieved and up to 11.1 L/kg after prolonged dosing.

Ohmefentanyl is an extremely potent opioid analgesic drug which selectively binds to the µ-opioid receptor.

Hydroxypethidine (Bemidone) is an opioid analgesic that is an analogue of the more commonly used pethidine (meperidine). Hydroxypethidine is slightly more potent than meperidine as an analgesic, 1.5x meperidine in potency, and it also has NMDA antagonist properties like its close relative ketobemidone.
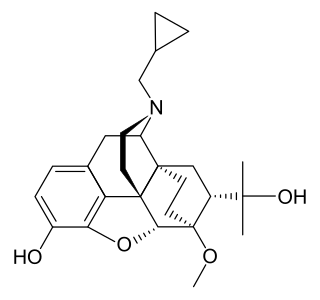
Diprenorphine, also known as diprenorfin, is a non-selective, high-affinity, weak partial agonist of the μ- (MOR), κ- (KOR), and δ-opioid receptor (DOR) which is used in veterinary medicine as an opioid antagonist. It is used to reverse the effects of super-potent opioid analgesics such as etorphine and carfentanil that are used for tranquilizing large animals. The drug is not approved for use in humans.

Oripavine is an opioid and the major metabolite of thebaine. It is the parent compound from which a series of semi-synthetic opioids are derived, which includes the compounds etorphine and buprenorphine. Although its analgesic potency is comparable to morphine, it is not used clinically due to its severe toxicity and low therapeutic index. Due to its use in manufacture of strong opioids, oripavine is a controlled substance in some jurisdictions.
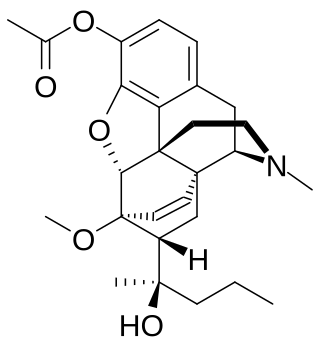
Acetorphine is a potent opioid analgesic, up to 8700 times stronger than morphine by weight. It is a derivative of the more well-known opioid etorphine, which is used as a very potent veterinary painkiller and anesthetic medication, primarily for the sedation of large animals such as elephants, giraffes and rhinos.
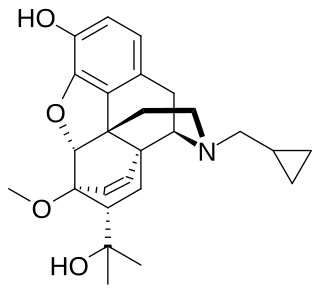
Cyprenorphine (M285), N-cyclo-propylmethyl-6,14-endoetheno-7α-(1-hydroxy-1-methylethyl)-6,7,8,14-tetrahydronororipavine, is an opioid drug. It is related to more well-known opioids such as buprenorphine, which is used as an analgesic and for the treatment of opioid addiction, and diprenorphine, which is used as an antidote to reverse the effects of other opioids. It is roughly 35 times as strong as nalorphine.

Prodine is an opioid analgesic that is an analog of pethidine (meperidine). It was developed in Germany in the late 1940s.
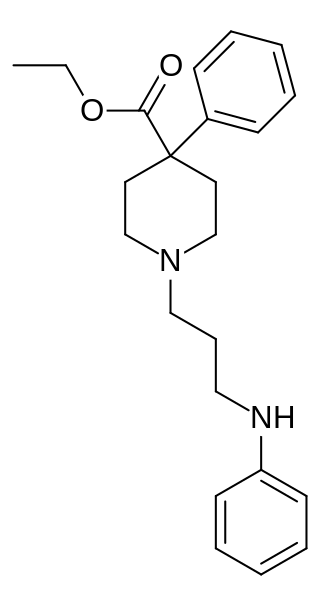
Piminodine (Alvodine) is an opioid analgesic that is an analogue of pethidine (meperidine). It was used in medicine briefly during the 1960s and 70s, but has largely fallen out of clinical use. It was used particularly for obstetric analgesia and in dental procedures and, like pethidine, could be combined with hydroxyzine to intensify the effects. The duration of action is 2–4 hours; 7.5–10 mg via the subcutaneous route is the most common starting dose, being equal to 80–100 mg of pethidine, 40–60 mg of alphaprodine and 10 mg of morphine. Oral formulations were also available.

Phenazocine is an opioid analgesic drug, which is related to pentazocine and has a similar profile of effects.

7-PET is an opioid analgesic drug that has 300 times the potency of morphine by weight. It was discovered by K.W. Bentley and is related to the more well known oripavine derivative etorphine, which is used as a veterinary painkiller and anesthetic medication for the sedation of large animals such as elephants, giraffes, and rhinos. 7-PET itself has a 3-O-methyl ether which reduces potency, but the 3-OH derivative is around 2200 times more potent than morphine, almost the same potency as etorphine as a μ agonist, and unexpectedly the 3-hydrogen compound is also around the same potency of 2000 times morphine.
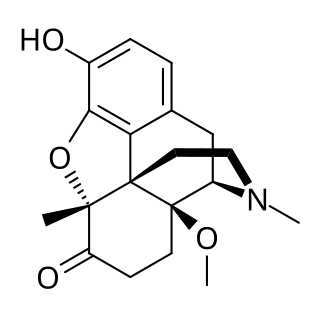
14-Methoxymetopon is an experimental opioid drug developed by a team led by Professor Helmut Schmidhammer at the University of Innsbruck in the mid-1990s. It is a derivative of metopon in which a methoxy group has been inserted at the 14-position. It is a highly potent analgesic drug that is around 500 times stronger than morphine when administered systemically; however, when given spinally or supraspinally, it exhibits analgesic activity up to a million fold greater than morphine. It binds strongly to the μ-opioid receptor and activates it to a greater extent than most similar opioid drugs. This produces an unusual pharmacological profile, and although 14-methoxymetopon acts as a potent μ-opioid full agonist in regard to some effects such as analgesia, a ceiling effect is seen on other effects such as constipation and respiratory depression which is believed to involve interaction with the κ-opioid receptor

14-Phenylpropoxymetopon (PPOM) is an opioid analogue that is a derivative of metopon which has been substituted with a γ-phenylpropoxy group at the 14-position. PPOM is a highly potent analgesic drug several thousand times stronger than morphine, with an even higher in vivo potency than etorphine. The 14-phenylpropoxy substitution appears to confer potent μ-opioid agonist activity, even when combined with substitutions such as N-cyclopropyl or N-allyl, which normally result in μ-opioid antagonist compounds.

BU-48 is a drug that is used in scientific research. It is from the oripavine family, related to better-known drugs such as etorphine and buprenorphine.

JWH-051 is an analgesic drug which is a cannabinoid agonist. Its chemical structure is closely related to that of the potent cannabinoid agonist HU-210, with the only difference being the removal of the hydroxyl group at position 1 of the aromatic ring. It was discovered and named after John W. Huffman.

Dextrallorphan (DXA) is a chemical of the morphinan class that is used in scientific research. It acts as a σ1 receptor agonist and NMDA receptor antagonist. It has no significant affinity for the σ2, μ-opioid, or δ-opioid receptor, or for the serotonin or norepinephrine transporter. As an NMDA receptor antagonist, in vivo, it is approximately twice as potent as dextromethorphan, and five-fold less potent than dextrorphan.