
Phenytoin (PHT), sold under the brand name Dilantin among others, is an anti-seizure medication. It is useful for the prevention of tonic-clonic seizures and focal seizures, but not absence seizures. The intravenous form, fosphenytoin, is used for status epilepticus that does not improve with benzodiazepines. It may also be used for certain heart arrhythmias or neuropathic pain. It can be taken intravenously or by mouth. The intravenous form generally begins working within 30 minutes and is effective for roughly 24 hours. Blood levels can be measured to determine the proper dose.
Anticonvulsants are a diverse group of pharmacological agents used in the treatment of epileptic seizures. Anticonvulsants are also increasingly being used in the treatment of bipolar disorder and borderline personality disorder, since many seem to act as mood stabilizers, and for the treatment of neuropathic pain. Anticonvulsants suppress the excessive rapid firing of neurons during seizures. Anticonvulsants also prevent the spread of the seizure within the brain.

Potassium bromide (KBr) is a salt, widely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries, with over-the-counter use extending to 1975 in the US. Its action is due to the bromide ion. Potassium bromide is used as a veterinary drug, as an antiepileptic medication for dogs.

Lamotrigine, sold under the brand name Lamictal among others, is a medication used to treat epilepsy and stabilize mood in bipolar disorder. For epilepsy, this includes focal seizures, tonic-clonic seizures, and seizures in Lennox-Gastaut syndrome. In bipolar disorder, lamotrigine has not been shown to reliably treat acute depression; but for patients with bipolar disorder who are not currently symptomatic, it appears to be effective in reducing the risk of future episodes of depression.

Oxcarbazepine, sold under the brand name Trileptal among others, is a medication used to treat epilepsy. For epilepsy it is used for both focal seizures and generalized seizures. It has been used both alone and as add-on therapy in people with bipolar disorder who have had no success with other treatments. It is taken by mouth.

Clonazepam, sold under the brand names Klonopin and Rivotril, is a medication used to prevent and treat seizures, panic disorder, anxiety disorders, and the movement disorder known as akathisia. It is a tranquilizer of the benzodiazepine class. It possesses anxiolytic, anticonvulsant, sedative, hypnotic, and skeletal muscle relaxant properties. It is typically taken by mouth. Effects begin within one hour and last between six and twelve hours.

Valpromide is a carboxamide derivative of valproic acid used in the treatment of epilepsy and some affective disorders. It is rapidly metabolised (80%) to valproic acid but has anticonvulsant properties itself. It may produce more stable plasma levels than valproic acid or sodium valproate and may be more effective at preventing febrile seizures. However, it is over one hundred times more potent as an inhibitor of liver microsomal epoxide hydrolase. This makes it incompatible with carbamazepine and can affect the ability of the body to remove other toxins. Valpromide is no safer during pregnancy than valproic acid.
Hydantoin, or glycolylurea, is a heterocyclic organic compound with the formula CH2C(O)NHC(O)NH. It is a colorless solid that arises from the reaction of glycolic acid and urea. It is an oxidized derivative of imidazolidine. In a more general sense, hydantoins can refer to a groups and a class of compounds with the same ring structure as the parent. For example, phenytoin (mentioned below) has two phenyl groups substituted onto the number 5 carbon in a hydantoin molecule.

Status epilepticus (SE), or status seizure, is a single seizure lasting more than 5 minutes or 2 or more seizures within a 5-minute period without the person returning to normal between them. Previous definitions used a 30-minute time limit. The seizures can be of the tonic–clonic type, with a regular pattern of contraction and extension of the arms and legs, or of types that do not involve contractions, such as absence seizures or complex partial seizures. Status epilepticus is a life-threatening medical emergency, particularly if treatment is delayed.
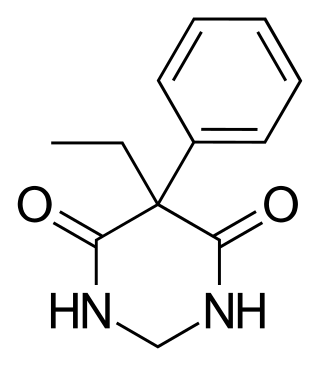
Primidone, sold under various brand names, is a barbiturate medication that is used to treat partial and generalized seizures, as well as essential tremors. It is taken by mouth.

Fosphenytoin, also known as fosphenytoin sodium, and sold under the brand name Cerebyx among others, is a water-soluble phenytoin prodrug that is administered intravenously to deliver phenytoin, potentially more safely than intravenous phenytoin. It is used in the acute treatment of convulsive status epilepticus.

Beclamide is a drug that possesses anticonvulsant activity. It is no longer used.
Ethotoin is an anticonvulsant drug used in the treatment of epilepsy. It is a hydantoin, similar to phenytoin. It is not available in the United States.

Metharbital was patented in 1905 by Emil Fischer working for Merck. It was marketed as Gemonil by Abbott Laboratories. It is a barbiturate anticonvulsant, used in the treatment of epilepsy. It has similar properties to phenobarbital.
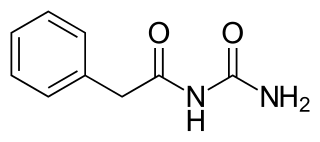
Phenacemide, also known as phenylacetylurea, is an anticonvulsant of the ureide (acetylurea) class. It is a congener and ring-opened analogue of phenytoin, and is structurally related to the barbiturates and to other hydantoins. Phenacemide was introduced in 1949 for the treatment of epilepsy, but was eventually withdrawn due to toxicity.
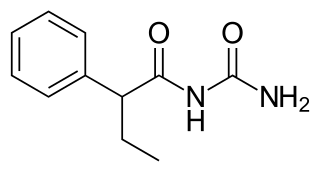
Pheneturide, also known as phenylethylacetylurea, is an anticonvulsant of the ureide class. Conceptually, it can be formed in the body as a metabolic degradation product from phenobarbital. It is considered to be obsolete and is now seldom used. It is marketed in Europe, including in Poland, Spain and the United Kingdom. Pheneturide has a similar profile of anticonvulsant activity and toxicity relative to phenacemide. As such, it is only used in cases of severe epilepsy when other, less-toxic drugs have failed. Pheneturide inhibits the metabolism and thus increases the levels of other anticonvulsants, such as phenytoin.

γ-Amino-β-hydroxybutyric acid (GABOB), also known as β-hydroxy-γ-aminobutyric acid (β-hydroxy-GABA), and sold under the brand name Gamibetal among others, is an anticonvulsant which is used for the treatment of epilepsy in Europe, Japan, and Mexico. It is a GABA analogue, or an analogue of the neurotransmitter γ-aminobutyric acid (GABA), and has been found to be an endogenous metabolite of GABA.

Remacemide is a drug which acts as a low-affinity NMDA antagonist with sodium channel blocking properties. It has been studied for the treatment of acute ischemic stroke, epilepsy, Huntington's disease, and Parkinson's disease.

Nirvanol, also known as ethylphenylhydantoin, is a derivative of hydantoin with anticonvulsant properties. Its 5-ethyl-5-phenyl substitution pattern is similar to that of phenobarbital. It is useful in the treatment of chorea.

Imepitoin (INN), sold under the brand name Pexion, is an anticonvulsant which is used in veterinary medicine in Europe to treat epilepsy in dogs. It was recently approved in the United States. The drug also has anxiolytic effects. It was originally developed to treat epilepsy in humans, but clinical trials were terminated upon findings of unfavorable metabolic differences in smokers and non-smokers.


















