
Paraponera clavata, commonly known as the bullet ant, is a species of ant named for its extremely painful sting. It inhabits humid lowland rainforests in Central and South America.

The Schmidt sting pain index is a pain scale rating the relative pain caused by different hymenopteran stings. It is mainly the work of Justin O. Schmidt, a former entomologist at the Carl Hayden Bee Research Center in Arizona. Schmidt published a number of works on the subject, and claimed to have been stung by the majority of stinging Hymenoptera.

Delta atracotoxin is a low-molecular-weight neurotoxic polypeptide found in the venom of the Sydney funnel-web spider.
Tityustoxin is a toxin found in the venom of scorpions from the subfamily Tityinae. By binding to voltage-dependent sodium ion channels and potassium channels, they cause sialorrhea, lacrimation and rhinorrhea.

Agatoxins are a class of chemically diverse polyamine and peptide toxins which are isolated from the venom of various spiders. Their mechanism of action includes blockade of glutamate-gated ion channels, voltage-gated sodium channels, or voltage-dependent calcium channels. Agatoxin is named after the funnel web spider which produces a venom containing several agatoxins. There are different agatoxins. The ω-agatoxins are approximately 100 amino acids in length and are antagonists of voltage-sensitive calcium channels and also block the release of neurotransmitters. For instance, the ω-agatoxin 1A is a selective blocker and will block L-type calcium channels whereas the ω-agatoxin 4B will inhibit voltage sensitive P-type calcium channels. The μ-agatoxins only act on insect voltage-gated sodium channels.
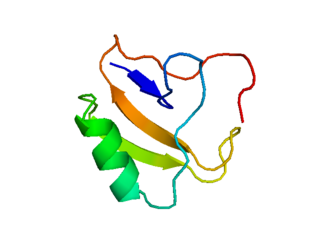
Scorpion toxins are proteins found in the venom of scorpions. Their toxic effect may be mammal- or insect-specific and acts by binding with varying degrees of specificity to members of the Voltage-gated ion channel superfamily; specifically, voltage-gated sodium channels, voltage-gated potassium channels, and Transient Receptor Potential (TRP) channels. The result of this action is to activate or inhibit the action of these channels in the nervous and cardiac organ systems. For instance, α-scorpion toxins MeuNaTxα-12 and MeuNaTxα-13 from Mesobuthus eupeus are neurotoxins that target voltage-gated Na+ channels (Navs), inhibiting fast inactivation. In vivo assays of MeuNaTxα-12 and MeuNaTxα-13 effects on mammalian and insect Navs show differential potency. These recombinants exhibit their preferential affinity for mammalian and insect Na+ channels at the α-like toxins' active site, site 3, in order to inactivate the cell membrane depolarization faster[6]. The varying sensitivity of different Navs to MeuNaTxα-12 and MeuNaTxα-13 may be dependent on the substitution of a conserved Valine residue for a Phenylalanine residue at position 1630 of the LD4:S3-S4 subunit or due to various changes in residues in the LD4:S5-S6 subunit of the Navs. Ultimately, these actions can serve the purpose of warding off predators by causing pain or to subdue predators.
Pompilidotoxins (PMTXs) are toxic substances that can only be found in the venom of several solitary wasps. This kind of wasp uses their venom to offensively capture prey and is relatively harmless to humans. This is in stark contrast to social insects that defend themselves and their colonies with their venom.
Birtoxin is a neurotoxin from the venom of the South African Spitting scorpion. By changing sodium channel activation, the toxin promotes spontaneous and repetitive firing much like pyrethroid insecticides do
BmKAEP is a neurotoxin from the venom of the Manchurian scorpion (Mesobuthus martensii). It is a β-toxin, which shift the activation voltage of sodium channels towards more negative potentials.

Paraponera is a genus of ants and the only genus in the subfamily Paraponerinae. The name means "near-Ponera".
Halcurin is a polypeptide neurotoxin from the sea anemone Halcurias sp. Based on sequence homology to type 1 and type 2 sea anemone toxins it is thought to delay channel inactivation by binding to the extracellular site 3 on the voltage gated sodium channels in a membrane potential-dependent manner.

Pi3 toxin is a purified peptide derivative of the Pandinus imperator scorpion venom. It is a potent blocker of voltage-gated potassium channel, Kv1.3 and is closely related to another peptide found in the venom, Pi2.
Beta-mammal toxin Cn2, also known as Cn2 toxin, is a single chain β-scorpion neurotoxic peptide and the primary toxin in the venom of the Centruroides noxius Hoffmann scorpion. The toxin specifically targets mammalian Nav1.6 voltage-gated sodium channels (VGSC).
LqhIT2 is a long-chain scorpion depressant β-toxin derived from Leiurus quinquestriatus hebraeus. It targets insect voltage-gated sodium channels (Navs) and shifts the voltage dependence of channel activation to a more negative membrane potential.
LmαTX5 is an α-scorpion toxin which inhibits the fast inactivation of voltage-gated sodium channels. It has been identified through transcriptome analysis of the venom gland of Lychas mucronatus, also known as the Chinese swimming scorpion – a scorpion species which is widely distributed in Southeast Asia.
DKK-SP1 is one of the many neurotoxins present in the scorpion Mesobuthus martensii. This toxin inhibits the voltage-gated sodium channel Nav1.8.
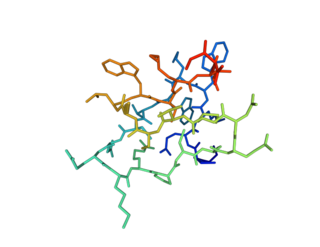
GiTx1 (β/κ-theraphotoxin-Gi1a) is a peptide toxin present in the venom of Grammostola iheringi. It reduces both inward and outward currents by blocking voltage-gated sodium and potassium channels, respectively.
Protoxin-I, also known as ProTx-I, or Beta/omega-theraphotoxin-Tp1a, is a 35-amino-acid peptide neurotoxin extracted from the venom of the tarantula Thrixopelma pruriens. Protoxin-I belongs to the inhibitory cystine knot (ICK) family of peptide toxins, which have been known to potently inhibit voltage-gated ion channels. Protoxin-I selectively blocks low voltage threshold T-type calcium channels, voltage gated sodium channels and the nociceptor cation channel TRPA1. Due to its unique ability to bind to TRPA1, Protoxin-I has been implicated as a valuable pharmacological reagent with potential applications in clinical contexts with regards to pain and inflammation

Delta hexatoxin Hv1 is a neurotoxic component found in the venom of the Australian funnel web spider.

Grammostola mechanotoxin #4, also known as M-theraphotoxin-Gr1a (M-TRTX-Gr1a), is a neurotoxin isolated from the venom of the spider Chilean rose tarantula Grammostola spatulate. This amphiphilic peptide, which consists of 35 amino acids, belongs to the inhibitory cysteine knot (ICK) peptide family. It reduces mechanical sensation by inhibiting mechanosensitive channels (MSCs).












