Vascular dementia (VaD) is dementia caused by problems in the blood supply to the brain, resulting from a cerebrovascular disease. Restricted blood supply (ischemia) leads to cell and tissue death in the affected region, known as an infarct. The three types of vascular dementia are subcortical vascular dementia, multi-infarct dementia, and stroke related dementia. Subcortical vascular dementia is brought about by damage to the small blood vessels in the brain. Multi-infarct dementia is brought about by a series of mini-strokes where many regions have been affected. The third type is stroke related where more serious damage may result. Such damage leads to varying levels of cognitive decline. When caused by mini-strokes, the decline in cognition is gradual. When due to a stroke, the cognitive decline can be traced back to the event.

Elan Corporation plc was a major drugs firm based in Dublin, Ireland, which had major interests in the United States. It was listed on the New York Stock Exchange as ELN, the Irish Stock Exchange as ELN.I, and the London Stock Exchange as ELN.L. In 2013, the company merged with Perrigo to form Perrigo Company PLC.

Donepezil, sold under the brand name Aricept among others, is a medication used to treat dementia of the Alzheimer's type. It appears to result in a small benefit in mental function and ability to function. Use, however, has not been shown to change the progression of the disease. Treatment should be stopped if no benefit is seen. It is taken by mouth or via a transdermal patch.

Memantine is a medication used to slow the progression of moderate-to-severe Alzheimer's disease. It is taken by mouth.

Moyamoya disease is a disease in which certain arteries in the brain are constricted. Blood flow is blocked by constriction and blood clots (thrombosis). A collateral circulation develops around the blocked vessels to compensate for the blockage, but the collateral vessels are small, weak, and prone to bleeding, aneurysm and thrombosis. On conventional angiography, these collateral vessels have the appearance of a "puff of smoke".

Cerebral hypoxia is a form of hypoxia, specifically involving the brain; when the brain is completely deprived of oxygen, it is called cerebral anoxia. There are four categories of cerebral hypoxia; they are, in order of increasing severity: diffuse cerebral hypoxia (DCH), focal cerebral ischemia, cerebral infarction, and global cerebral ischemia. Prolonged hypoxia induces neuronal cell death via apoptosis, resulting in a hypoxic brain injury.

A watershed stroke is defined as a brain ischemia that is localized to the vulnerable border zones between the tissues supplied by the anterior, posterior and middle cerebral arteries. The actual blood stream blockage/restriction site can be located far away from the infarcts. Watershed locations are those border-zone regions in the brain supplied by the major cerebral arteries where blood supply is decreased. Watershed strokes are a concern because they comprise approximately 10% of all ischemic stroke cases. The watershed zones themselves are particularly susceptible to infarction from global ischemia as the distal nature of the vasculature predisposes these areas to be most sensitive to profound hypoperfusion.
Bapineuzumab is a humanized monoclonal antibody that acts on the nervous system and may have potential therapeutic value for the treatment of Alzheimer's disease and possibly glaucoma. However, in 2012 it failed to produce significant cognitive improvements in patients in two major trials, despite lowering key biomarkers of AD, amyloid brain plaque and hyperphosphorylated tau protein in CSF.

The Roskamp Institute, was co-founded by Robert and Diane Roskamp, and Fiona Crawford and Michael Mullan in Sarasota, Florida in 2003. It is a nonprofit biomedical research facility specializing neurological research including Alzheimer's disease, traumatic brain injury, Gulf War syndrome, and posttraumatic stress disorder. It also operates an onsite neurology clinic. The institute is focused on finding the causes and treatments for neuropsychiatric and neurodegenerative diseases.

Latrepirdine is an antihistamine drug which has been used clinically in Russia since 1983.
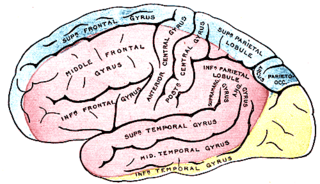
Anterior cerebral artery syndrome is a condition whereby the blood supply from the anterior cerebral artery (ACA) is restricted, leading to a reduction of the function of the portions of the brain supplied by that vessel: the medial aspects of the frontal and parietal lobes, basal ganglia, anterior fornix and anterior corpus callosum.
Lipohyalinosis is a cerebral small vessel disease affecting the small arteries, arterioles or capillaries in the brain. Originally defined by C. Miller Fisher as 'segmental arteriolar wall disorganisation', it is characterized by vessel wall thickening and a resultant reduction in luminal diameter. Fisher considered this small vessel disease to be the result of hypertension, induced in the acute stage by fibrinoid necrosis that would lead to occlusion and hence lacunar stroke. However, recent evidence suggests that endothelial dysfunction as a result of inflammation is a more likely cause for it. This may occur subsequent to blood–brain barrier failure, and lead to extravasation of serum components into the brain that are potentially toxic. Lacunar infarction could thus occur in this way, and the narrowing – the hallmark feature of lipohyalinosis – may merely be a feature of the swelling occurring around it that squeezes on the structure.

Alzheimer's disease (AD) is a neurodegenerative disease that usually starts slowly and progressively worsens, and is the cause of 60–70% of cases of dementia. The most common early symptom is difficulty in remembering recent events. As the disease advances, symptoms can include problems with language, disorientation, mood swings, loss of motivation, self-neglect, and behavioral issues. As a person's condition declines, they often withdraw from family and society. Gradually, bodily functions are lost, ultimately leading to death. Although the speed of progression can vary, the typical life expectancy following diagnosis is three to nine years.
Solanezumab is a monoclonal antibody being investigated by Eli Lilly as a neuroprotector for patients with Alzheimer's disease. The drug originally attracted extensive media coverage proclaiming it a breakthrough, but it has failed to show promise in Phase III trials.

Rivastigmine is a cholinesterase inhibitor used for the treatment of mild to moderate Alzheimer's disease. The drug can be administered orally or via a transdermal patch; the latter form reduces the prevalence of side effects, which typically include nausea and vomiting.
Crenezumab is a fully humanized monoclonal antibody against human 1-40 and 1-42 beta amyloid, which is being investigated as a treatment of Alzheimer's disease. Crenezumab is highly homologous to solanezumab, another monoclonal antibody targeting amyloid-β peptides. In June 2022, the US National Institutes of Health announced that the drug failed as a medication for early-onset Alzheimer's disease following the results of a decade-long clinical trial.

Verubecestat (MK-8931) was an experimental drug for the treatment of Alzheimer's disease. It is an inhibitor of beta-secretase 1 (BACE1), which, after initial promise proved disappointing.
Lecanemab, sold under the brand name Leqembi, is a monoclonal antibody medication used for the treatment of Alzheimer's disease. Lecanemab is an amyloid beta-directed antibody. It is given via intravenous infusion. The most common side effects of lecanemab include headache, infusion-related reactions and amyloid-related imaging abnormalities, a side effect known to occur with the class of antibodies targeting amyloid.

Phenserine is a synthetic drug which has been investigated as a medication to treat Alzheimer's disease (AD), as the drug exhibits neuroprotective and neurotrophic effects.

Simufilam (PTI-125) is an Investigational New Drug for the treatment of Alzheimer's disease. It is being developed by the American pharmaceutical firm Cassava Sciences. The drug is in phase III clinical trials as of October 2023. There are two phase III clinical studies: RETHINK-ALZ, a 52-week trial, is set to complete in 2024, and REFOCUS-ALZ, spanning 76 weeks, is projected to finish in 2025.














