
The areca nut is the fruit of the areca palm, which grows in much of the tropical Pacific, South Asia, Southeast Asia, and parts of east Africa. It is commonly referred to as betel nut, not to be confused with betel leaves that are often used to wrap it.

The betel, Piper betle, is a species of flowering plant in the pepper family Piperaceae, native to Southeast Asia. It is an evergreen, dioecious vine, with glossy heart-shaped leaves and white catkins. Betel plants are cultivated for their leaves which are most commonly used as flavoring in chewing areca nut.

Betel nut chewing, also called betel quid chewing or areca nut chewing, is a practice in which areca nuts are chewed together with slaked lime and betel leaves for their stimulant and narcotic effects. The practice is widespread in Southeast Asia, Micronesia, Island Melanesia, and South Asia. It is also found among both Han Chinese immigrants and indigenous peoples of Taiwan, Madagascar, and parts of southern China. It has also been introduced to the Caribbean in colonial times.

Noscapine is a benzylisoquinoline alkaloid, of the phthalideisoquinoline structural subgroup, which has been isolated from numerous species of the family Papaveraceae. It lacks significant hypnotic, euphoric, or analgesic effects affording it with very low addictive potential. This agent is primarily used for its antitussive (cough-suppressing) effects.

Areca catechu is a species of palm which grows in much of the tropical Pacific, Asia, and parts of east Africa. The palm is native to the Philippines, but is widespread in cultivation and is considered naturalized in Malaysia, Indonesia, New Guinea, Taiwan, Madagascar, Cambodia, Laos, Myanmar, Thailand, Vietnam, southern China, India, Nepal, Bangladesh, the Maldives, Sri Lanka, parts of the Pacific Islands, and also in the West Indies.

Arecoline is a nicotinic acid-based mild parasympathomimetic stimulant alkaloid found in the areca nut, the fruit of the areca palm. It is an odourless oily liquid. It can bring a sense of enhanced alertness and energy along with mild feelings of euphoria and relaxation. The psychoactive effects are comparable to that of nicotine.

Reuptake inhibitors (RIs) are a type of reuptake modulators. It is a drug that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.

Nipecotic acid is a GABA uptake inhibitor used in scientific research.

Amfonelic acid is a research chemical and dopaminergic stimulant with antibiotic properties. Although limited clinical trials have been conducted, it's primarily used in scientific research.

Tandamine is a selective norepinephrine reuptake inhibitor with a tricyclic structure. It was developed in the 1970s as an antidepressant but was never commercialized. Tandamine is analogous to pirandamine, which, instead, acts as a selective serotonin reuptake inhibitor (SSRI).

(–)-2β-Carbomethoxy-3β-(4-bromophenyl)tropane is a semi-synthetic alkaloid in the phenyltropane group of psychostimulant compounds. First publicized in the 1990s, it has not been used enough to have gained a fully established profile. RTI-51 can be expected to have properties lying somewhere in between RTI-31 and RTI-55. It has a ratio of monoamine reuptake inhibition of dopamine > serotonin > norepinephrine which is an unusual balance of effects not produced by other commonly used compounds. It has been used in its 76Br radiolabelled form to map the distribution of dopamine transporters in the brain.
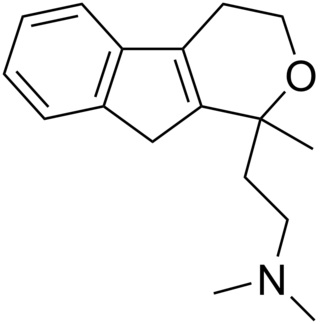
Pirandamine (AY-23,713) is a tricyclic derivative which acts as a selective serotonin reuptake inhibitor (SSRI). It was investigated in the 1970s as a potential antidepressant but clinical development was not commenced and it was never marketed. Pirandamine is structurally related to tandamine, which, in contrast, is a selective norepinephrine reuptake inhibitor.
The endocannabinoid transporters (eCBTs) are transport proteins for the endocannabinoids. Most neurotransmitters are water-soluble and require transmembrane proteins to transport them across the cell membrane. The endocannabinoids on the other hand, are non-charged lipids that readily cross lipid membranes. However, since the endocannabinoids are water immiscible, protein transporters have been described that act as carriers to solubilize and transport the endocannabinoids through the aqueous cytoplasm. These include the heat shock proteins (Hsp70s) and fatty acid-binding proteins for anandamide (FABPs). FABPs such as FABP1, FABP3, FABP5, and FABP7 have been shown to bind endocannabinoids. FABP inhibitors attenuate the breakdown of anandamide by the enzyme fatty acid amide hydrolase (FAAH) in cell culture. One of these inhibitors (SB-FI-26), isolated from a virtual library of a million compounds, belongs to a class of compounds that act as an anti-nociceptive agent with mild anti-inflammatory activity in mice. These truxillic acids and their derivatives have been known to have anti-inflammatory and anti-nociceptive effects in mice and are active components of a Chinese herbal medicine used to treat rheumatism and pain in human. The blockade of anandamide transport may, at least in part, be the mechanism through which these compounds exert their anti-nociceptive effects.

Guvacine is an hydrogenated pyridine alkaloid found in areca nuts. It is the N-demethylated derivative of arecaidine and the product of ester hydrolysis of guvacoline, both of which are also found in areca nuts as well. It is also an inhibitor of gamma-aminobutyric acid uptake. Lime is said to hydrolyse guvacoline to guvacine.

CI-966 (developmental code name) is a central nervous system depressant acting as a GABA reuptake inhibitor, specifically a highly potent and selective blocker of the GABA transporter 1 (GAT-1) (IC50 = 0.26 μM), and hence indirect and non-selective GABA receptor full agonist. It was investigated as a potential anticonvulsant, anxiolytic, and neuroprotective therapeutic but was discontinued during clinical development due to the incidence of severe adverse effects at higher doses and hence was never marketed.

Alstonine is an indoloquinolizidine alkaloid and putative antipsychotic constituent of various plant species including Alstonia boonei, Catharanthus roseus, Picralima nitida, Rauwolfia caffra and Rauwolfia vomitoria. In preclinical studies alstonine attenuates MK-801-induced hyperlocomotion, working memory deficit and social withdrawal. It also possesses anxiolytic-like effects in preclinical studies, attenuates amphetamine-induced lethality and stereotypy as well as apomorphine-induced stereotypy, and attenuates haloperidol-induced catalepsy. These effects appear to be mediated by stimulation of the 5-HT2C receptor. In addition, alstonine, similarly to clozapine, indirectly inhibits the reuptake of glutamate in hippocampal slices. Unlike clozapine however, the effect of which is abolished by the D2 receptor agonist apomorphine, alstonine requires 5-HT2A and 5-HT2C receptors to produce this effect, as it is abolished by antagonists of these receptors. Also unlike clozapine, alstonine lacks pro-convulsant activity in mice.

Areca nut production in India is dominant in the coastal region within 400 kilometres (250 mi) from the coast line, and also in some other non-coastal states of India. Areca nut, a tropical crop, is popularly known as betel nut, as its common usage in the country is for mastication with betel leaves. It is a palm tree species under the family of Arecaceae. It has commercial and economic importance not only in India but also in China and Southeast Asia.

Areca alkaloids are a group of piperidine alkaloids found in the areca nut, the seeds of the areca palm.

















