
Hydromorphone, also known as dihydromorphinone, and sold under the brand name Dilaudid among others, is a morphinan opioid used to treat moderate to severe pain. Typically, long-term use is only recommended for pain due to cancer. It may be used by mouth or by injection into a vein, muscle, or under the skin. Effects generally begin within half an hour and last for up to five hours. A 2016 Cochrane review found little difference in benefit between hydromorphone and other opioids for cancer pain.
In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base. An alternative name is chlorhydrate, which comes from French. An archaic alternative name is muriate, derived from hydrochloric acid's ancient name: muriatic acid.

Promethazine, sold under the brand name Phenergan among others, is a first-generation antihistamine, antipsychotic, sedative, and antiemetic used to treat allergies, insomnia, and nausea. It may also help with some symptoms associated with the common cold and may also be used for sedating people who are agitated or anxious, an effect that has led to some recreational use. Promethazine is taken by mouth (oral), as a rectal suppository, or by injection into a muscle (IM).

Dihydrocodeine is a semi-synthetic opioid analgesic prescribed for pain or severe dyspnea, or as an antitussive, either alone or compounded with paracetamol (acetaminophen) or aspirin. It was developed in Germany in 1908 and first marketed in 1911.
Sominex is the trademarked name for several over the counter sleep aids.
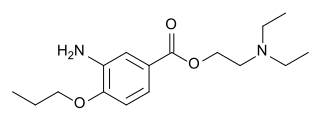
Proxymetacaine (INN) or proparacaine (USAN) is a topical anesthetic drug of the aminoester group.

Propiomazine, sold under the brand name Propavan among others, is an antihistamine which is used to treat insomnia and to produce sedation and relieve anxiety before or during surgery or other procedures and in combination with analgesics as well as during labor. Propiomazine is a phenothiazine, but is not used therapeutically as a neuroleptic because it does not block dopamine receptors well.

Tricyclics are cyclic chemical compounds that contain three fused rings of atoms.

Lean or purple drank is a polysubstance drink used as a recreational drug. It is prepared by mixing prescription-grade cough or cold syrup containing an opioid drug and an anti-histamine drug with a soft drink and sometimes hard candy. The beverage originated in Houston as early as the 1960s and is popular in hip hop culture, especially within the Southern United States. Codeine/promethazine syrup is usually used to make lean, but other syrups are also used.

Thebacon, or dihydrocodeinone enol acetate, is a semisynthetic opioid that is similar to hydrocodone and is most commonly synthesised from thebaine. Thebacon was invented in Germany in 1924, four years after the first synthesis of hydrocodone. Thebacon is a derivative of acetyldihydrocodeine, where only the 6–7 double bond is saturated. Thebacon is marketed as its hydrochloride salt under the trade name Acedicon, and as its bitartrate under Diacodin and other trade names. The hydrochloride salt has a free base conversion ratio of 0.846. Other salts used in research and other settings include thebacon's phosphate, hydrobromide, citrate, hydroiodide, and sulfate.

8-Chlorotheophylline, also known as 1,3-dimethyl-8-chloroxanthine, is a stimulant drug of the xanthine chemical class, with physiological effects similar to caffeine. Its main use is in combination (salt) with diphenhydramine in the antiemetic dimenhydrinate (Dramamine). Diphenhydramine reduces nausea but causes drowsiness, and the stimulant properties of 8-Chlorotheophylline help reduce that side effect.

Isothipendyl is a 1st generation H1 antagonist (antihistamine) and anticholinergic used as an antipruritic. In the 2020s, at least, it is rarely used in the first line relief of allergies due to the anticholinergic side effect of somnolence but does have some limited use through topical application in the relief of insect bites and related itching (pruritus).
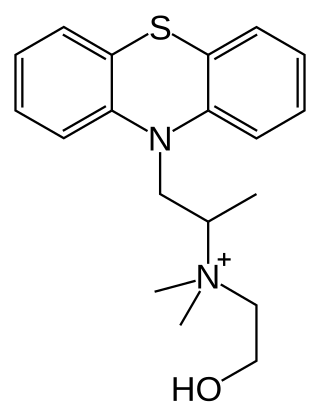
Hydroxyethylpromethazine is an antihistamine with anticholinergic properties. It is structurally analogous to promethazine.

Dimenoxadol (INN), or dimenoxadole (BAN), is an opioid analgesic which is a benzilic acid derivative, closely related to benactyzine. Further, the structure is similar to methadone and related compounds like dextropropoxyphene.

Indeloxazine (INN) is an antidepressant and cerebral activator that was marketed in Japan and South Korea by Yamanouchi Pharmaceutical Co., Ltd for the treatment of psychiatric symptoms associated with cerebrovascular diseases, namely depression resulting from stroke, emotional disturbance, and avolition. It was marketed from 1988 to 1998, when it was removed from the market reportedly for lack of effectiveness.
The drug combination prednisolone/promethazine is an antidote for snake bites.

Anpirtoline (chemical formula C10H13ClN2S) is a synthetic chemical compound used as a 5-HT1B receptor agonist as well as a 5-HT3 receptor antagonist, causing a decrease in serotonin synthesis and a reduction in aggressive behavior. Anpirtoline hydrochloride appears as a white solid and is soluble in water. Being synthetic, the compound can be purchased from suppliers.
An analgesic adjuvant is a medication that is typically used for indications other than pain control but provides control of pain (analgesia) in some painful diseases. This is often part of multimodal analgesia, where one of the intentions is to minimize the need for opioids.

Hypidone (developmental code name YL-0919) is an investigational serotonergic antidepressant which is under development for the treatment of major depressive disorder. It acts as a serotonin reuptake inhibitor, 5-HT1A receptor partial agonist, and 5-HT6 receptor full agonist. It is used as the hydrochloride salt. As of January 2021, hypidone is in phase 2 clinical trials for major depressive disorder.















