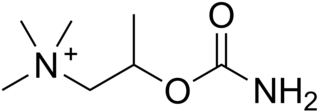
Bethanechol is a parasympathomimetic choline carbamate that selectively stimulates muscarinic receptors without any effect on nicotinic receptors. Unlike acetylcholine, bethanechol is not hydrolyzed by cholinesterase and will therefore have a long duration of action. Bethanechol is sold under the brand names Duvoid (Roberts), Myotonachol (Glenwood), Urecholine, and Urocarb (Hamilton). The name bethanechol refers to its structure as the urethane of beta-methylcholine.

Glycopyrronium bromide is a medication of the muscarinic anticholinergic group. It does not cross the blood–brain barrier and consequently has few to no central effects. It is given by mouth, via intravenous injection, on the skin, and via inhalation. It is a synthetic quaternary ammonium compound. The cation, which is the active moiety, is called glycopyrronium (INN) or glycopyrrolate (USAN).

Tolterodine, sold under the brand name Detrol among others, is a medication used to treat frequent urination, urinary incontinence, or urinary urgency. Effects are seen within an hour. It is taken by mouth.

Oxybutynin, sold as under the brand name Ditropan among others, is an anticholinergic drug primarily used to treat overactive bladder. It is widely considered a first-line therapy for overactive bladder due to its well-studied side effect profile, broad applicability, and continued efficacy over long periods of time. It works similar to tolterodine, darifenacin, and solifenacin, although it is usually preferred over these medications. It is sometimes used off-label for treatment of hyperhidrosis, or excessive sweating. It has also been used off-label to treat bed wetting in children, but this use has declined, as it is most likely ineffective in this role. It is taken by mouth or applied to the skin.

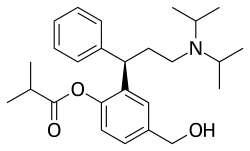
Darifenacin is a medication used to treat urinary incontinence due to an overactive bladder. It was discovered by scientists at the Pfizer research site in Sandwich, UK under the identifier UK-88,525 and used to be marketed by Novartis. In 2010, the US rights were sold to Warner Chilcott for US$400 million.

Solifenacin, sold as the brand name Vesicare among others, is a medicine used to treat overactive bladder and neurogenic detrusor overactivity (NDO). It may help with incontinence, urinary frequency, and urinary urgency.

Trospium chloride is a muscarinic antagonist used to treat overactive bladder. It has side effects typical of this class of drugs, namely dry mouth, stomach upset, and constipation; these side effects cause problems with people taking their medicine as directed. However it doesn't cause central nervous system side effects like some other muscarinic antagonists. It is in pregnancy category C and is excreted in breast milk.

Overactive bladder (OAB) is a common condition where there is a frequent feeling of needing to urinate to a degree that it negatively affects a person's life. The frequent need to urinate may occur during the day, at night, or both. Loss of bladder control may occur with this condition. This condition is also sometimes characterized by a sudden and involuntary contraction of the bladder muscles, in response to excitement or anticipation. This in turn leads to a frequent and urgent need to urinate.

Silodosin, sold under the brand name Urief among others, is a medication for the symptomatic treatment of benign prostatic hyperplasia. It acts as an alpha-1 adrenergic receptor antagonist.

Propiverine is an anticholinergic drug used for the treatment of urinary urgency, frequency and urge incontinence, all symptoms of overactive bladder syndrome. It is a muscarinic antagonist.

Dimethyl fumarate (DMF) is the methyl ester of fumaric acid and is named after the earth smoke plant. Dimethyl fumarate combined with three other fumaric acid esters (FAEs) is solely licensed in Germany as an oral therapy for psoriasis. Since 2013, it has been approved by the U.S. Food and Drug Administration (FDA) as a treatment option for adults with relapsing multiple sclerosis. In 2017, an oral formulation of dimethyl fumarate was approved for medical use in the European Union as a treatment for moderate-to-severe plaque psoriasis. Dimethyl fumarate is thought to have immunomodulatory properties without causing significant immunosuppression.
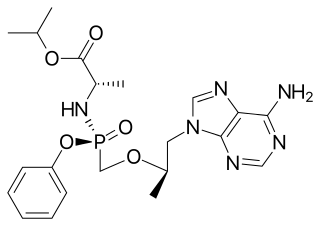
Tenofovir alafenamide, sold under the brand name Vemlidy, is an antiviral medication used against hepatitis B and HIV. It is used for the treatment of chronic hepatitis B virus (HBV) infection in adults with compensated liver disease and is given in combination with other medications for the prevention and treatment of HIV. It is taken by mouth.
Mirabegron, sold under the brand name Myrbetriq among others, is a medication used to treat overactive bladder. Its benefits are similar to antimuscarinic medication such as solifenacin or tolterodine. It is taken by mouth.
Aclidinium bromide/formoterol, sold under the brand names Duaklir and Brimica, is a fixed-dose combination medication for inhalation, used in the management of chronic obstructive pulmonary disease (COPD). It consists of aclidinium bromide, a long-acting muscarinic antagonist, and formoterol, a long-acting β2 agonist.

Rimegepant, sold under the brand name Nurtec ODT among others, is a medication used for the acute treatment of migraine with or without aura in adults and the prophylactic/ preventive treatment of episodic migraine in adults. It is taken by mouth to dissolve on or under the tongue. It works by blocking CGRP receptors.
Beclometasone/formoterol/glycopyrronium, sold under the brand name Trimbow among others, is an inhalable fixed-dose combination medication for the treatment of chronic obstructive pulmonary disease (COPD) and asthma. It contains beclometasone dipropionate, formoterol fumarate dihydrate, and glycopyrronium bromide.
Autonomic drugs can either inhibit or enhance the functions of the parasympathetic and sympathetic nervous systems. This type of drug can be used to treat a wide range of diseases, such as glaucoma, asthma, urinary, gastrointestinal and cardiopulmonary disorders.
Glycopyrronium bromide/formoterol, sold under the brand name Bevespi Aerosphere, is a combination medication for the maintenance treatment of chronic obstructive pulmonary disease (COPD). It is a combination of glycopyrronium bromide and formoterol. It is inhaled.

Vibegron, sold under the brand name Gemtesa, is a medication for the treatment of overactive bladder. Vibegron is a selective beta-3 adrenergic receptor agonist.

Relugolix/estradiol/norethisterone acetate, sold under the brand names Myfembree and Ryeqo, is a fixed-dose combination hormonal medication which is used for the treatment of heavy menstrual bleeding associated with uterine leiomyomas (fibroids) and for moderate to severe pain associated with endometriosis. It contains relugolix, an orally active gonadotropin-releasing hormone antagonist, estradiol, an estrogen, and norethisterone acetate, a progestin. The medication is taken by mouth.