
Chlorphenamine, also known as chlorpheniramine, is an antihistamine used to treat the symptoms of allergic conditions such as allergic rhinitis. It is taken by mouth. The medication takes effect within two hours and lasts for about 4-6.
A parasympathomimetic drug, sometimes called a cholinomimetic drug or cholinergic receptor stimulating agent, is a substance that stimulates the parasympathetic nervous system (PSNS). These chemicals are also called cholinergic drugs because acetylcholine (ACh) is the neurotransmitter used by the PSNS. Chemicals in this family can act either directly by stimulating the nicotinic or muscarinic receptors, or indirectly by inhibiting cholinesterase, promoting acetylcholine release, or other mechanisms. Common uses of parasympathomimetics include glaucoma, sjögren syndrome and underactive bladder.
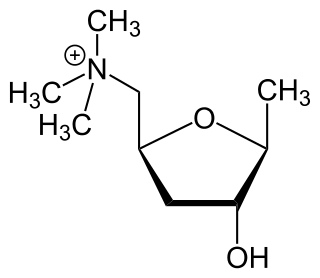
Muscarinic acetylcholine receptors, or mAChRs, are acetylcholine receptors that form G protein-coupled receptor complexes in the cell membranes of certain neurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibers in the parasympathetic nervous system.

A muscarinic agonist is an agent that activates the activity of the muscarinic acetylcholine receptor. The muscarinic receptor has different subtypes, labelled M1-M5, allowing for further differentiation.
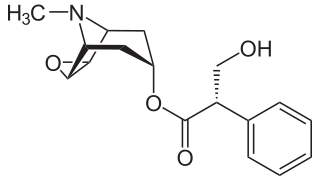
A muscarinic receptor antagonist (MRA) is a type of anticholinergic agent that blocks the activity of the muscarinic acetylcholine receptor. The muscarinic receptor is a protein involved in the transmission of signals through certain parts of the nervous system, and muscarinic receptor antagonists work to prevent this transmission from occurring. Notably, muscarinic antagonists reduce the activation of the parasympathetic nervous system. The normal function of the parasympathetic system is often summarised as "rest-and-digest", and includes slowing of the heart, an increased rate of digestion, narrowing of the airways, promotion of urination, and sexual arousal. Muscarinic antagonists counter this parasympathetic "rest-and-digest" response, and also work elsewhere in both the central and peripheral nervous systems.

The human muscarinic acetylcholine receptor M5, encoded by the CHRM5 gene, is a member of the G protein-coupled receptor superfamily of integral membrane proteins. It is coupled to Gq protein. Binding of the endogenous ligand acetylcholine to the M5 receptor triggers a number of cellular responses such as adenylate cyclase inhibition, phosphoinositide degradation, and potassium channel modulation. Muscarinic receptors mediate many of the effects of acetylcholine in the central and peripheral nervous system. The clinical implications of this receptor have not been fully explored; however, stimulation of this receptor is known to effectively decrease cyclic AMP levels and downregulate the activity of protein kinase A (PKA).

The muscarinic acetylcholine receptor M1, also known as the cholinergic receptor, muscarinic 1, is a muscarinic receptor that in humans is encoded by the CHRM1 gene. It is localized to 11q13.

The muscarinic acetylcholine receptor M2, also known as the cholinergic receptor, muscarinic 2, is a muscarinic acetylcholine receptor that in humans is encoded by the CHRM2 gene. Multiple alternatively spliced transcript variants have been described for this gene.

The muscarinic acetylcholine receptor, also known as cholinergic/acetylcholine receptor M3, or the muscarinic 3, is a muscarinic acetylcholine receptor encoded by the human gene CHRM3.

The muscarinic acetylcholine receptor M4, also known as the cholinergic receptor, muscarinic 4 (CHRM4), is a protein that, in humans, is encoded by the CHRM4 gene.

Himbacine is an alkaloid isolated from the bark of Australian magnolias. Himbacine has been synthesized using a Diels-Alder reaction as a key step. Himbacine's activity as a muscarinic receptor antagonist, with specificity for the muscarinic acetylcholine receptor M2, made it a promising starting point in Alzheimer's disease research. The development of a muscarinic antagonist based on himbacine failed but an analog, vorapaxar, has been approved by the FDA as a thrombin receptor antagonist.

Xanomeline is a small molecule muscarinic acetylcholine receptor agonist that was first synthesized in a collaboration between Eli Lilly and Novo Nordisk as an investigational therapeutic being studied for the treatment of central nervous system disorders.

Vedaclidine (INN, codenamed LY-297,802, NNC 11-1053) is an experimental analgesic drug which acts as a mixed agonist–antagonist at muscarinic acetylcholine receptors, being a potent and selective agonist for the M1 and M4 subtypes, yet an antagonist at the M2, M3 and M5 subtypes. It is orally active and an effective analgesic over 3× the potency of morphine, with side effects such as salivation and tremor only occurring at many times the effective analgesic dose. Human trials showed little potential for development of dependence or abuse, and research is continuing into possible clinical application in the treatment of neuropathic pain and cancer pain relief.

Oxaprotiline, also known as hydroxymaprotiline, is a norepinephrine reuptake inhibitor of the tetracyclic antidepressant (TeCA) family that is related to maprotiline. Though investigated as an antidepressant, it was never marketed.
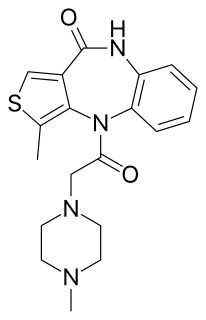
Telenzepine is a thienobenzodiazepine acting as selective M1 antimuscarinic. It is used in the treatment of peptic ulcers. Telenzepine is atropisomeric, in other words the molecule has a stereogenic C–N-axis. In neutral aqueous solution it displays a half-life for racemization of the order of 1000 years. The enantiomers have been resolved. The activity is related to the (+)-isomer which is about 500-fold more active than the (–)-isomer at muscarinic receptors in the rat cerebral cortex.
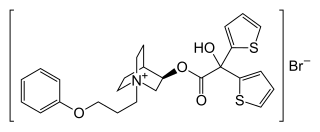
Aclidinium bromide (INN) is a long-acting, inhaled muscarinic antagonist (LAMA) approved in the United States on July 24, 2012 as a maintenance treatment for chronic obstructive pulmonary disease (COPD).

PD-102,807 is a drug which acts as a selective antagonist for the muscarinic acetylcholine receptor M4. It is used in scientific research for studying the effects of the different muscarinic receptor subtypes in the body and brain.
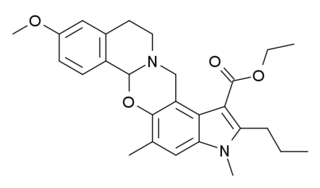
PD-0298029 is a drug which acts as a selective antagonist for the muscarinic acetylcholine receptor M4. It was developed for the treatment of Parkinson's disease, but poor bioavailability and rapid metabolism in animal studies have meant its use is largely limited to in vitro research into the M4 and other muscarinic receptors.

AFDX-384 (BIBN-161) is a drug which acts as a selective antagonist of the muscarinic acetylcholine receptors, with selectivity for the M2 and M4 subtypes. It is used mainly for mapping the distribution of M2 and M4 muscarinic receptors in the brain, and studying their involvement in the development and treatment of dementia and schizophrenia.

4-DAMP (1,1-dimethyl-4-diphenylacetoxypiperidinium iodide) is a selective muscarinic acetylcholine receptor (mAChR) M3 antagonist. It is also able to antagonize M1 receptors but "prefers" M3. It competitively binds to the acetylcholine binding site on mAChRs, causing right-ward shift in the dose response curves for mAChR agonists.


















