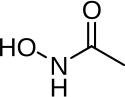
Beta-lactamases are enzymes produced by bacteria that provide multi-resistance to beta-lactam antibiotics such as penicillins, cephalosporins, cephamycins, monobactams and carbapenems (ertapenem), although carbapenems are relatively resistant to beta-lactamase. Beta-lactamase provides antibiotic resistance by breaking the antibiotics' structure. These antibiotics all have a common element in their molecular structure: a four-atom ring known as a beta-lactam (β-lactam) ring. Through hydrolysis, the enzyme lactamase breaks the β-lactam ring open, deactivating the molecule's antibacterial properties.
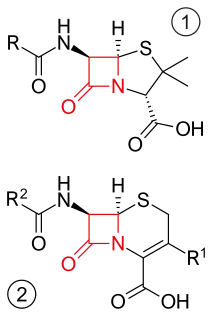
β-lactam antibiotics are antibiotics that contain a beta-lactam ring in their chemical structure. This includes penicillin derivatives (penams), cephalosporins and cephamycins (cephems), monobactams, carbapenems and carbacephems. Most β-lactam antibiotics work by inhibiting cell wall biosynthesis in the bacterial organism and are the most widely used group of antibiotics. Until 2003, when measured by sales, more than half of all commercially available antibiotics in use were β-lactam compounds. The first β-lactam antibiotic discovered, penicillin, was isolated from a rare variant of Penicillium notatum.
Ureases, functionally, belong to the superfamily of amidohydrolases and phosphotriesterases. Ureases are found in numerous bacteria, fungi, algae, plants, and some invertebrates, as well as in soils, as a soil enzyme. They are nickel-containing metalloenzymes of high molecular weight.

Nitrification is the biological oxidation of ammonia to nitrite followed by the oxidation of the nitrite to nitrate occurring through separate organisms or direct ammonia oxidation to nitrate in comammox bacteria. The transformation of ammonia to nitrite is usually the rate limiting step of nitrification. Nitrification is an important step in the nitrogen cycle in soil. Nitrification is an aerobic process performed by small groups of autotrophic bacteria and archaea.

Omeprazole, sold under the brand names Prilosec and Losec, among others, is a medication used in the treatment of gastroesophageal reflux disease (GERD), peptic ulcer disease, and Zollinger–Ellison syndrome. It is also used to prevent upper gastrointestinal bleeding in people who are at high risk. Omeprazole is a proton-pump inhibitor (PPI) and its effectiveness is similar to other PPIs. It can be taken by mouth or by injection into a vein.

Cytochromes P450 (CYPs) are a superfamily of enzymes containing heme as a cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various compounds, as well as for hormone synthesis and breakdown. In 1963, Estabrook, Cooper, and Rosenthal described the role of CYP as a catalyst in steroid hormone synthesis and drug metabolism. In plants, these proteins are important for the biosynthesis of defensive compounds, fatty acids, and hormones.

Clavulanic acid is a β-lactam drug that functions as a mechanism-based β-lactamase inhibitor. While not effective by itself as an antibiotic, when combined with penicillin-group antibiotics, it can overcome antibiotic resistance in bacteria that secrete β-lactamase, which otherwise inactivates most penicillins.

Enoxolone is a pentacyclic triterpenoid derivative of the beta-amyrin type obtained from the hydrolysis of glycyrrhizic acid, which was obtained from the herb liquorice. It is used in flavoring and it masks the bitter taste of drugs like aloe and quinine. It is effective in the treatment of peptic ulcer and also has expectorant (antitussive) properties. It has some additional pharmacological properties with possible antiviral, antifungal, antiprotozoal, and antibacterial activities.

In biochemistry, suicide inhibition, also known as suicide inactivation or mechanism-based inhibition, is an irreversible form of enzyme inhibition that occurs when an enzyme binds a substrate analog and forms an irreversible complex with it through a covalent bond during the normal catalysis reaction. The inhibitor binds to the active site where it is modified by the enzyme to produce a reactive group that reacts irreversibly to form a stable inhibitor-enzyme complex. This usually uses a prosthetic group or a coenzyme, forming electrophilic alpha and beta unsaturated carbonyl compounds and imines.

An enzyme inhibitor is a molecule that binds to an enzyme and decreases its activity. By binding to enzymes' active sites, inhibitors reduce the compatibility of substrate and enzyme and this leads to the inhibition of Enzyme-Substrate complexes' formation, preventing the catalysis of reactions and decreasing the amount of product produced by a reaction. It can be said that as the concentration of enzyme inhibitors increases, the rate of enzyme activity decreases, and thus, the amount of product produced is inversely proportional to the concentration of inhibitor molecules. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used in pesticides. Not all molecules that bind to enzymes are inhibitors; enzyme activators bind to enzymes and increase their enzymatic activity, while enzyme substrates bind and are converted to products in the normal catalytic cycle of the enzyme.

Nitisinone, sold under the brand name Orfadin among others, is a medication used to slow the effects of hereditary tyrosinemia type 1 (HT-1).

The acetolactate synthase (ALS) enzyme is a protein found in plants and micro-organisms. ALS catalyzes the first step in the synthesis of the branched-chain amino acids.

Beta-lactamases are a family of enzymes involved in bacterial resistance to beta-lactam antibiotics. They act by breaking the beta-lactam ring that allows penicillin-like antibiotics to work. Strategies for combating this form of resistance have included the development of new beta-lactam antibiotics that are more resistant to cleavage and the development of the class of enzyme inhibitors called beta-lactamase inhibitors. Although β-lactamase inhibitors have little antibiotic activity of their own, they prevent bacterial degradation of beta-lactam antibiotics and thus extend the range of bacteria the drugs are effective against.
In enzymology, glutamate racemase is an enzyme that catalyzes the chemical reaction

Antifolates are a class of antimetabolite medications that antagonise (that is, block) the actions of folic acid (vitamin B9). Folic acid's primary function in the body is as a cofactor to various methyltransferases involved in serine, methionine, thymidine and purine biosynthesis. Consequently, antifolates inhibit cell division, DNA/RNA synthesis and repair and protein synthesis. Some such as proguanil, pyrimethamine and trimethoprim selectively inhibit folate's actions in microbial organisms such as bacteria, protozoa and fungi. The majority of antifolates work by inhibiting dihydrofolate reductase (DHFR).

Salicylhydroxamic acid is a drug that is a potent and irreversible enzyme inhibitor of the urease enzyme in various bacteria and plants; it is usually used for urinary tract infections. The molecule is similar to urea but is not hydrolyzable by urease; it thus disrupts the bacteria's metabolism through competitive inhibition. It is also a trypanocidal agent. When administered orally, it is metabolized to salicylamide, which exerts analgesic, antipyretic, and anti-inflammatory effects.

Pracinostat (SB939) is an orally bioavailable, small-molecule histone deacetylase (HDAC) inhibitor based on hydroxamic acid with potential anti-tumor activity characterized by favorable physicochemical, pharmaceutical, and pharmacokinetic properties.

Nitrapyrin is an organic compound with the formula ClC5H3NCCl3. It is a widely used nitrification inhibitor in agriculture as well as a soil bactericide and has been in use since 1974. Nitrapyrin was put up for review by the EPA and deemed safe for use in 2005. Since nitrapyrin is an effective nitrification inhibitor to the bacteria Nitrosomonas it has been shown to drastically the reduce NO2 emissions of soil. Nitrapyrin is a white crystalline solid with a sweet odor and is often mixed with anhydrous ammonia for application.
Competitive inhibition is interruption of a chemical pathway owing to one chemical substance inhibiting the effect of another by competing with it for binding or bonding. Any metabolic or chemical messenger system can potentially be affected by this principle, but several classes of competitive inhibition are especially important in biochemistry and medicine, including the competitive form of enzyme inhibition, the competitive form of receptor antagonism, the competitive form of antimetabolite activity, and the competitive form of poisoning.

Furegrelate, also known as 5-(3-pyridinylmethyl)benzofurancarboxylic acid, is a chemical compound with thromboxane enzyme inhibiting properties that was originally developed by Pharmacia Corporation as a drug to treat arrhythmias, ischaemic heart disorders, and thrombosis but was discontinued. It is commercially available in the form furegrelate sodium salt.