
Cysteine is a semiessential proteinogenic amino acid with the formula HOOC−CH(−NH2)−CH2−SH. The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. Cysteine is chiral, but interestingly, both D and L-cysteine are found in nature with D-cysteine having been found in developing brain.
Etanercept, sold under the brand name Enbrel among others, is a biologic medical product that is used to treat autoimmune diseases by interfering with tumor necrosis factor (TNF), a soluble inflammatory cytokine, by acting as a TNF inhibitor. It has US Food and Drug Administration (FDA) approval to treat rheumatoid arthritis, juvenile idiopathic arthritis and psoriatic arthritis, plaque psoriasis and ankylosing spondylitis. Tumor necrosis factor alpha (TNFα) is the "master regulator" of the inflammatory (immune) response in many organ systems. Autoimmune diseases are caused by an overactive immune response. Etanercept has the potential to treat these diseases by inhibiting TNF-alpha.

Ritonavir, sold under the brand name Norvir, is an antiretroviral medication used along with other medications to treat HIV/AIDS. This combination treatment is known as highly active antiretroviral therapy (HAART). Ritonavir is a protease inhibitor, though it now mainly serves to boost the potency of other protease inhibitors. It may also be used in combination with other medications to treat hepatitis C and COVID-19. It is taken by mouth. Tablets of ritonavir are not bioequivalent to capsules, as the tablets may result in higher peak plasma concentrations.

Cystinuria is an inherited autosomal recessive disease characterized by high concentrations of the amino acid cystine in the urine, leading to the formation of cystine stones in the kidneys, ureters, and bladder. It is a type of aminoaciduria. "Cystine", not "cysteine," is implicated in this disease; the former is a dimer of the latter.
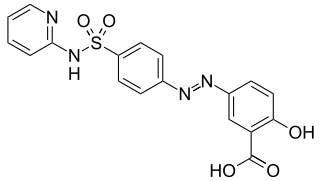
Sulfasalazine, sold under the brand name Azulfidine among others, is a medication used to treat rheumatoid arthritis, ulcerative colitis, and Crohn's disease. It is considered by some to be a first-line treatment in rheumatoid arthritis. It is taken by mouth or can be administered rectally.

Piroxicam is a nonsteroidal anti-inflammatory drug (NSAID) of the oxicam class used to relieve the symptoms of painful inflammatory conditions like arthritis. Piroxicam works by preventing the production of endogenous prostaglandins which are involved in the mediation of pain, stiffness, tenderness and swelling. The medicine is available as capsules, tablets and as a prescription-free gel 0.5%. It is also available in a betadex formulation, which allows a more rapid absorption of piroxicam from the digestive tract. Piroxicam is one of the few NSAIDs that can be given parenteral routes.
Adalimumab, sold under the brand name Humira and others, is a disease-modifying antirheumatic drug and monoclonal antibody used to treat rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease, ulcerative colitis, plaque psoriasis, hidradenitis suppurativa, and uveitis. It is administered by subcutaneous injection. It works by inactivating tumor necrosis factor-alpha (TNFα).

Penicillamine, sold under the brand name of Cuprimine among others, is a medication primarily used for the treatment of Wilson's disease. It is also used for people with kidney stones who have high urine cystine levels, rheumatoid arthritis, and various heavy metal poisonings. It is taken by mouth.

Hydroxychloroquine, sold under the brand name Plaquenil among others, is a medication used to prevent and treat malaria in areas where malaria remains sensitive to chloroquine. Other uses include treatment of rheumatoid arthritis, lupus, and porphyria cutanea tarda. It is taken by mouth, often in the form of hydroxychloroquine sulfate.
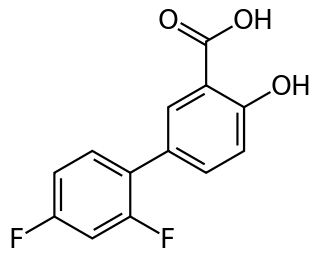
Diflunisal is a salicylic acid derivative with analgesic and anti-inflammatory activity. It was developed by Merck Sharp & Dohme in 1971, as MK647, after showing promise in a research project studying more potent chemical analogs of aspirin. It was first sold under the brand name Dolobid, marketed by Merck & Co., but generic versions are now widely available. It is classed as a nonsteroidal anti-inflammatory drug (NSAID) and is available in 250 mg and 500 mg tablets.

Pyrimethamine, sold under the brand name Daraprim among others, is a medication used with leucovorin to treat the parasitic diseases toxoplasmosis and cystoisosporiasis. It is also used with dapsone as a second-line option to prevent Pneumocystis jiroveci pneumonia in people with HIV/AIDS. It was previously used for malaria but is no longer recommended due to resistance. Pyrimethamine is taken by mouth.

Certolizumab pegol, sold under the brand name Cimzia, is a biopharmaceutical medication for the treatment of Crohn's disease, rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. It is a fragment of a monoclonal antibody specific to tumor necrosis factor alpha (TNF-α) and is manufactured by UCB.
Tocilizumab, sold under the brand name Actemra among others, is an immunosuppressive drug, used for the treatment of rheumatoid arthritis, systemic juvenile idiopathic arthritis, a severe form of arthritis in children, and COVID‑19. It is a humanized monoclonal antibody against the interleukin-6 receptor (IL-6R). Interleukin 6 (IL-6) is a cytokine that plays an important role in immune response and is implicated in the pathogenesis of many diseases, such as autoimmune diseases, multiple myeloma and prostate cancer. Tocilizumab was jointly developed by Osaka University and Chugai, and was licensed in 2003 by Hoffmann-La Roche.

Teriflunomide, sold under the brand name Aubagio, is the active metabolite of leflunomide. Teriflunomide was investigated in the Phase III clinical trial TEMSO as a medication for multiple sclerosis (MS). The study was completed in July 2010. 2-year results were positive. However, the subsequent TENERE head-to-head comparison trial reported that "although permanent discontinuations [of therapy] were substantially less common among MS patients who received teriflunomide compared with interferon beta-1a, relapses were more common with teriflunomide." The drug was approved for use in the United States in September 2012 and for use in the European Union in August 2013.
A Janus kinase inhibitor, also known as JAK inhibitor or jakinib, is a type of immune modulating medication, which inhibits the activity of one or more of the Janus kinase family of enzymes, thereby interfering with the JAK-STAT signaling pathway in lymphocytes.

Tofacitinib, sold under the brand Xeljanz among others, is a medication used to treat rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, polyarticular course juvenile idiopathic arthritis, and ulcerative colitis. It is a janus kinase (JAK) inhibitor, discovered and developed by the National Institutes of Health and Pfizer.
Sarilumab, sold under the brand name Kevzara, is a human monoclonal antibody medication against the interleukin-6 receptor. Regeneron Pharmaceuticals and Sanofi developed the drug for the treatment of rheumatoid arthritis (RA), for which it received US FDA approval on 22 May 2017 and European Medicines Agency approval on 23 June 2017.

Filgotinib, sold under the brand name Jyseleca, is a medication used for the treatment of rheumatoid arthritis (RA). It was developed by the Belgian-Dutch biotech company Galapagos NV.
Harrow Health, formerly known as Imprimis Pharmaceuticals, is a publicly traded pharmaceutical company based in Nashville, TN.

Upadacitinib, sold under the brand name Rinvoq, is a medication used for the treatment of rheumatoid arthritis, psoriatic arthritis, atopic dermatitis, ulcerative colitis, Crohn's disease, ankylosing spondylitis, and axial spondyloarthritis. Upadacitinib is a Janus kinase (JAK) inhibitor that works by blocking the action of enzymes called Janus kinases. These enzymes are involved in setting up processes that lead to inflammation, and blocking their effect brings inflammation in the joints under control.















