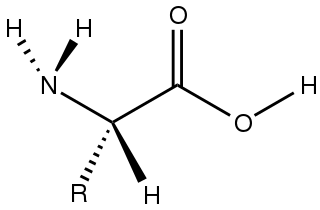
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 appear in the genetic code of life.

Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid (carbamic acid is unstable). In the gas phase, it is a molecule with the chemical formula NH2‐CH2‐COOH. In solution or in the solid, glycine exists as the zwitterion. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine is integral to the formation of alpha-helices in secondary protein structure due to the "flexibility" caused by such a small R group. Glycine is also an inhibitory neurotransmitter – interference with its release within the spinal cord (such as during a Clostridium tetani infection) can cause spastic paralysis due to uninhibited muscle contraction.

Phenylalanine is an essential α-amino acid with the formula C
9H
11NO
2. It can be viewed as a benzyl group substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. This essential amino acid is classified as neutral, and nonpolar because of the inert and hydrophobic nature of the benzyl side chain. The L-isomer is used to biochemically form proteins coded for by DNA. Phenylalanine is a precursor for tyrosine, the monoamine neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), and the biological pigment melanin. It is encoded by the messenger RNA codons UUU and UUC.

Aspartic acid, is an α-amino acid that is used in the biosynthesis of proteins. The L-isomer of aspartic acid is one of the 22 proteinogenic amino acids, i.e., the building blocks of proteins. D-aspartic acid is one of two D-amino acids commonly found in mammals. Apart from a few rare exceptions, D-aspartic acid is not used for protein synthesis but is incorporated into some peptides and plays a role as a neurotransmitter/neuromodulator.

Dihydrofolate reductase, or DHFR, is an enzyme that reduces dihydrofolic acid to tetrahydrofolic acid, using NADPH as an electron donor, which can be converted to the kinds of tetrahydrofolate cofactors used in 1-carbon transfer chemistry. In humans, the DHFR enzyme is encoded by the DHFR gene. It is found in the q14.1 region of chromosome 5.

(2S,4R)-4-Hydroxyproline, or L-hydroxyproline (C5H9O3N), is an amino acid, abbreviated as Hyp or O, e.g., in Protein Data Bank.

Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) amino acids, 20 in the standard genetic code and an additional 2 that can be incorporated by special translation mechanisms.
In biochemistry, biotinylation is the process of covalently attaching biotin to a protein, nucleic acid or other molecule. Biotinylation is rapid, specific and is unlikely to disturb the natural function of the molecule due to the small size of biotin. Biotin binds to streptavidin and avidin with an extremely high affinity, fast on-rate, and high specificity, and these interactions are exploited in many areas of biotechnology to isolate biotinylated molecules of interest. Biotin-binding to streptavidin and avidin is resistant to extremes of heat, pH and proteolysis, making capture of biotinylated molecules possible in a wide variety of environments. Also, multiple biotin molecules can be conjugated to a protein of interest, which allows binding of multiple streptavidin, avidin or neutravidin protein molecules and increases the sensitivity of detection of the protein of interest. There is a large number of biotinylation reagents available that exploit the wide range of possible labelling methods. Due to the strong affinity between biotin and streptavidin, the purification of biotinylated proteins has been a widely used approach to identify protein-protein interactions and post-translational events such as ubiquitylation in molecular biology.
In molecular biology, biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism.
Affinity chromatography is a method of separating a biomolecule from a mixture, based on a highly specific macromolecular binding interaction between the biomolecule and another substance. The specific type of binding interaction depends on the biomolecule of interest; antigen and antibody, enzyme and substrate, receptor and ligand, or protein and nucleic acid binding interactions are frequently exploited for isolation of various biomolecules. Affinity chromatography is useful for its high selectivity and resolution of separation, compared to other chromatographic methods.
Cyanogen bromide is the inorganic compound with the formula (CN)Br or BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides, and synthesize other compounds. The compound is classified as a pseudohalogen.

MES (2-(N-morpholino)ethanesulfonic acid) is a chemical compound that contains a morpholine ring. It has a molecular weight of 195.2 g/mol and the chemical formula is C6H13NO4S. Synonyms include: 2-morpholinoethanesulfonic acid; 2-(4-morpholino)ethanesulfonic acid; 2-(N-morpholino)ethanesulfonic acid; 2-(4-morpholino)ethanesulfonic acid; MES; MES hydrate; and morpholine-4-ethanesulfonic acid hydrate. MOPS is a similar pH buffering compound which contains a propanesulfonic moiety instead of an ethanesulfonic one.
Maltose-binding protein (MBP) is a part of the maltose/maltodextrin system of Escherichia coli, which is responsible for the uptake and efficient catabolism of maltodextrins. It is a complex regulatory and transport system involving many proteins and protein complexes. MBP has an approximate molecular mass of 42.5 kilodaltons.

Glycine N-methyltransferase is an enzyme that in humans is encoded by the GNMT gene.
An aldehyde tag is a short peptide tag that can be further modified to add fluorophores, glycans, PEG chains, or reactive groups for further synthesis. A short, genetically-encoded peptide with a consensus sequence LCxPxR is introduced into fusion proteins, and by subsequent treatment with the formylglycine-generating enzyme (FGE), the cysteine of the tag is converted to a reactive aldehyde group. This electrophilic group can be targeted by an array of aldehyde-specific reagents, such as aminooxy- or hydrazide-functionalized compounds.
CRM197 is a non-toxic mutant of diphtheria toxin, currently used as a carrier protein for polysaccharides and haptens to make them immunogenic. There is some dispute about the toxicity of CRM197, with evidence that it is toxic to yeast cells and some mammalian cell lines.
Dr. Herbert Weissbach NAS NAI AAM is an American biochemist/molecular biologist.
Modeccin is a toxic lectin, a group of glycoproteins capable of binding specifically to sugar moieties. Different toxic lectins are present in seeds of different origin. Modeccin is found in the roots of the African plant Adenia digitata. These roots are often mistaken for edible roots, which has led to some cases of intoxication. Sometimes the fruit is eaten, or a root extract is drunk as a manner of suicide.
Charles Clifton Richardson is an American biochemist and professor at Harvard University. Richardson received his undergraduate education at Duke University, where he majored in medicine. He received his M.D. at Duke Medical School in 1960. Richardson works as a professor at Harvard Medical School, and he served as editor/associate editor of the Annual Review of Biochemistry from 1972 to 2003. Richardson received the American Chemical Society Award in Biological Chemistry in 1968, as well as numerous other accolades.











