The Beer-Lambert law is commonly applied to chemical analysis measurements to determine the concentration of chemical species that absorb light. It is often referred to as Beer's law. In physics, the Bouguer–Lambert law is an empirical law which relates the extinction or attenuation of light to the properties of the material through which the light is travelling. It had its first use in astronomical extinction. The fundamental law of extinction is sometimes called the Beer-Bouguer-Lambert law or the Bouguer-Beer-Lambert law or merely the extinction law. The extinction law is also used in understanding attenuation in physical optics, for photons, neutrons, or rarefied gases. In mathematical physics, this law arises as a solution of the BGK equation.
In physics, the cross section is a measure of the probability that a specific process will take place when some kind of radiant excitation intersects a localized phenomenon. For example, the Rutherford cross-section is a measure of probability that an alpha particle will be deflected by a given angle during an interaction with an atomic nucleus. Cross section is typically denoted σ (sigma) and is expressed in units of area, more specifically in barns. In a way, it can be thought of as the size of the object that the excitation must hit in order for the process to occur, but more exactly, it is a parameter of a stochastic process.

In physics, optical depth or optical thickness is the natural logarithm of the ratio of incident to transmitted radiant power through a material. Thus, the larger the optical depth, the smaller the amount of transmitted radiant power through the material. Spectral optical depth or spectral optical thickness is the natural logarithm of the ratio of incident to transmitted spectral radiant power through a material. Optical depth is dimensionless, and in particular is not a length, though it is a monotonically increasing function of optical path length, and approaches zero as the path length approaches zero. The use of the term "optical density" for optical depth is discouraged.

Radiometry is a set of techniques for measuring electromagnetic radiation, including visible light. Radiometric techniques in optics characterize the distribution of the radiation's power in space, as opposed to photometric techniques, which characterize the light's interaction with the human eye. The fundamental difference between radiometry and photometry is that radiometry gives the entire optical radiation spectrum, while photometry is limited to the visible spectrum. Radiometry is distinct from quantum techniques such as photon counting.

The reflectance of the surface of a material is its effectiveness in reflecting radiant energy. It is the fraction of incident electromagnetic power that is reflected at the boundary. Reflectance is a component of the response of the electronic structure of the material to the electromagnetic field of light, and is in general a function of the frequency, or wavelength, of the light, its polarization, and the angle of incidence. The dependence of reflectance on the wavelength is called a reflectance spectrum or spectral reflectance curve.

In physics, Planck's law describes the spectral density of electromagnetic radiation emitted by a black body in thermal equilibrium at a given temperature T, when there is no net flow of matter or energy between the body and its environment.

In optics, a Fabry–Pérot interferometer (FPI) or etalon is an optical cavity made from two parallel reflecting surfaces. Optical waves can pass through the optical cavity only when they are in resonance with it. It is named after Charles Fabry and Alfred Perot, who developed the instrument in 1899. Etalon is from the French étalon, meaning "measuring gauge" or "standard".

In optical physics, transmittance of the surface of a material is its effectiveness in transmitting radiant energy. It is the fraction of incident electromagnetic power that is transmitted through a sample, in contrast to the transmission coefficient, which is the ratio of the transmitted to incident electric field.
In radiometry, radiance is the radiant flux emitted, reflected, transmitted or received by a given surface, per unit solid angle per unit projected area. Radiance is used to characterize diffuse emission and reflection of electromagnetic radiation, and to quantify emission of neutrinos and other particles. The SI unit of radiance is the watt per steradian per square metre. It is a directional quantity: the radiance of a surface depends on the direction from which it is being observed.

In heat transfer, Kirchhoff's law of thermal radiation refers to wavelength-specific radiative emission and absorption by a material body in thermodynamic equilibrium, including radiative exchange equilibrium. It is a special case of Onsager reciprocal relations as a consequence of the time reversibility of microscopic dynamics, also known as microscopic reversibility.

The term quantum efficiency (QE) may apply to incident photon to converted electron (IPCE) ratio of a photosensitive device, or it may refer to the TMR effect of a magnetic tunnel junction.
In radiometry, radiant intensity is the radiant flux emitted, reflected, transmitted or received, per unit solid angle, and spectral intensity is the radiant intensity per unit frequency or wavelength, depending on whether the spectrum is taken as a function of frequency or of wavelength. These are directional quantities. The SI unit of radiant intensity is the watt per steradian, while that of spectral intensity in frequency is the watt per steradian per hertz and that of spectral intensity in wavelength is the watt per steradian per metre —commonly the watt per steradian per nanometre. Radiant intensity is distinct from irradiance and radiant exitance, which are often called intensity in branches of physics other than radiometry. In radio-frequency engineering, radiant intensity is sometimes called radiation intensity.
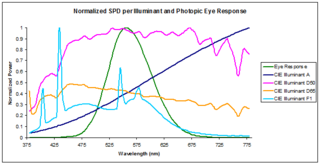
In radiometry, photometry, and color science, a spectral power distribution (SPD) measurement describes the power per unit area per unit wavelength of an illumination. More generally, the term spectral power distribution can refer to the concentration, as a function of wavelength, of any radiometric or photometric quantity.
An isotropic radiator is a theoretical point source of electromagnetic or sound waves which radiates the same intensity of radiation in all directions. It has no preferred direction of radiation. It radiates uniformly in all directions over a sphere centred on the source. Isotropic radiators are used as reference radiators with which other sources are compared, for example in determining the gain of antennas. A coherent isotropic radiator of electromagnetic waves is theoretically impossible, but incoherent radiators can be built. An isotropic sound radiator is possible because sound is a longitudinal wave.

In radiometry, radiant flux or radiant power is the radiant energy emitted, reflected, transmitted, or received per unit time, and spectral flux or spectral power is the radiant flux per unit frequency or wavelength, depending on whether the spectrum is taken as a function of frequency or of wavelength. The SI unit of radiant flux is the watt (W), one joule per second, while that of spectral flux in frequency is the watt per hertz and that of spectral flux in wavelength is the watt per metre —commonly the watt per nanometre.
In radiometry, radiosity is the radiant flux leaving a surface per unit area, and spectral radiosity is the radiosity of a surface per unit frequency or wavelength, depending on whether the spectrum is taken as a function of frequency or of wavelength. The SI unit of radiosity is the watt per square metre, while that of spectral radiosity in frequency is the watt per square metre per hertz (W·m−2·Hz−1) and that of spectral radiosity in wavelength is the watt per square metre per metre (W·m−3)—commonly the watt per square metre per nanometre. The CGS unit erg per square centimeter per second is often used in astronomy. Radiosity is often called intensity in branches of physics other than radiometry, but in radiometry this usage leads to confusion with radiant intensity.
The linear attenuation coefficient, attenuation coefficient, or narrow-beam attenuation coefficient characterizes how easily a volume of material can be penetrated by a beam of light, sound, particles, or other energy or matter. A coefficient value that is large represents a beam becoming 'attenuated' as it passes through a given medium, while a small value represents that the medium had little effect on loss. The SI unit of attenuation coefficient is the reciprocal metre (m−1). Extinction coefficient is another term for this quantity, often used in meteorology and climatology. Most commonly, the quantity measures the exponential decay of intensity, that is, the value of downward e-folding distance of the original intensity as the energy of the intensity passes through a unit thickness of material, so that an attenuation coefficient of 1 m−1 means that after passing through 1 metre, the radiation will be reduced by a factor of e, and for material with a coefficient of 2 m−1, it will be reduced twice by e, or e2. Other measures may use a different factor than e, such as the decadic attenuation coefficient below. The broad-beam attenuation coefficient counts forward-scattered radiation as transmitted rather than attenuated, and is more applicable to radiation shielding. The mass attenuation coefficient is the attenuation coefficient normalized by the density of the material.
In the study of heat transfer, absorptance of the surface of a material is its effectiveness in absorbing radiant energy. It is the ratio of the absorbed to the incident radiant power.
In radiometry, radiant exposure or fluence is the radiant energy received by a surface per unit area, or equivalently the irradiance of a surface, integrated over time of irradiation, and spectral exposure is the radiant exposure per unit frequency or wavelength, depending on whether the spectrum is taken as a function of frequency or of wavelength. The SI unit of radiant exposure is the joule per square metre, while that of spectral exposure in frequency is the joule per square metre per hertz and that of spectral exposure in wavelength is the joule per square metre per metre —commonly the joule per square metre per nanometre.
In chemistry, the molar absorption coefficient or molar attenuation coefficient is a measurement of how strongly a chemical species absorbs, and thereby attenuates, light at a given wavelength. It is an intrinsic property of the species. The SI unit of molar absorption coefficient is the square metre per mole, but in practice, quantities are usually expressed in terms of M−1⋅cm−1 or L⋅mol−1⋅cm−1. In older literature, the cm2/mol is sometimes used; 1 M−1⋅cm−1 equals 1000 cm2/mol. The molar absorption coefficient is also known as the molar extinction coefficient and molar absorptivity, but the use of these alternative terms has been discouraged by the IUPAC.




































































