
Acetylcholine (ACh) is an organic compound that functions in the brain and body of many types of animals as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Parts in the body that use or are affected by acetylcholine are referred to as cholinergic. Substances that increase or decrease the overall activity of the cholinergic system are called cholinergics and anticholinergics, respectively.

Atropine is a tropane alkaloid and anticholinergic medication used to treat certain types of nerve agent and pesticide poisonings as well as some types of slow heart rate, and to decrease saliva production during surgery. It is typically given intravenously or by injection into a muscle. Eye drops are also available which are used to treat uveitis and early amblyopia. The intravenous solution usually begins working within a minute and lasts half an hour to an hour. Large doses may be required to treat some poisonings.

Muscarine, L-(+)-muscarine, or muscarin is a natural product found in certain mushrooms, particularly in Inocybe and Clitocybe species, such as the deadly C. dealbata. Mushrooms in the genera Entoloma and Mycena have also been found to contain levels of muscarine which can be dangerous if ingested. Muscarine has been found in harmless trace amounts in Boletus, Hygrocybe, Lactarius and Russula. Trace concentrations of muscarine are also found in Amanita muscaria, though the pharmacologically more relevant compound from this mushroom is the Z-drug-like alkaloid muscimol. A. muscaria fruitbodies contain a variable dose of muscarine, usually around 0.0003% fresh weight. This is very low and toxicity symptoms occur very rarely. Inocybe and Clitocybe contain muscarine concentrations up to 1.6%.
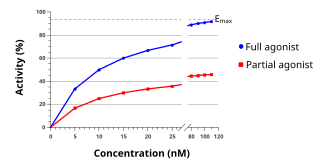
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist.
Anticholinergics are substances that block the action of the neurotransmitter called acetylcholine (ACh) at synapses in the central and peripheral nervous system.

Chlorphenamine, also known as chlorpheniramine, is an antihistamine used to treat the symptoms of allergic conditions such as allergic rhinitis. It is taken orally. The medication takes effect within two hours and lasts for about 4-6 hours.

Muscarinic acetylcholine receptors, or mAChRs, are acetylcholine receptors that form G protein-coupled receptor complexes in the cell membranes of certain neurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibers in the parasympathetic nervous system.

Epibatidine is a chlorinated alkaloid that is secreted by the Ecuadoran frog Epipedobates anthonyi and poison dart frogs from the Ameerega genus. It was discovered by John W. Daly in 1974, but its structure was not fully elucidated until 1992. Whether epibatidine is the first observed example of a chlorinated alkaloid remains controversial, due to challenges in conclusively identifying the compound from the limited samples collected by Daly. By the time that high-resolution spectrometry was used in 1991, there remained less than one milligram of extract from Daly's samples, raising concerns about possible contamination. Samples from other batches of the same species of frog failed to yield epibatidine.

Chlorprothixene, sold under the brand name Truxal among others, is a typical antipsychotic of the thioxanthene group.

Oxybutynin, sold as under the brand names Ditropan among others, is a medication used to treat overactive bladder. It works similar to tolterodine, Darifenacin, and Solifenacin. While used for bed wetting in children, evidence to support this use is poor. It is taken by mouth or applied to the skin.

Darifenacin is a medication used to treat urinary incontinence due to an overactive bladder. It was discovered by scientists at the Pfizer research site in Sandwich, UK under the identifier UK-88,525 and used to be marketed by Novartis. In 2010, the US rights were sold to Warner Chilcott for US$400 million.

Solifenacin, sold as the brand name Vesicare among others, is a medicine used to treat overactive bladder and neurogenic detrusor overactivity (NDO). It may help with incontinence, urinary frequency, and urinary urgency.
A muscarinic receptor antagonist (MRA) is a type of anticholinergic agent that blocks the activity of the muscarinic acetylcholine receptor. The muscarinic receptor is a protein involved in the transmission of signals through certain parts of the nervous system, and muscarinic receptor antagonists work to prevent this transmission from occurring. Notably, muscarinic antagonists reduce the activation of the parasympathetic nervous system. The normal function of the parasympathetic system is often summarised as "rest-and-digest", and includes slowing of the heart, an increased rate of digestion, narrowing of the airways, promotion of urination, and sexual arousal. Muscarinic antagonists counter this parasympathetic "rest-and-digest" response, and also work elsewhere in both the central and peripheral nervous systems.

The human muscarinic acetylcholine receptor M5, encoded by the CHRM5 gene, is a member of the G protein-coupled receptor superfamily of integral membrane proteins. It is coupled to Gq protein. Binding of the endogenous ligand acetylcholine to the M5 receptor triggers a number of cellular responses such as adenylate cyclase inhibition, phosphoinositide degradation, and potassium channel modulation. Muscarinic receptors mediate many of the effects of acetylcholine in the central and peripheral nervous system. The clinical implications of this receptor have not been fully explored; however, stimulation of this receptor is known to effectively decrease cyclic AMP levels and downregulate the activity of protein kinase A (PKA).

The muscarinic acetylcholine receptor M1, also known as the cholinergic receptor, muscarinic 1, is a muscarinic receptor that in humans is encoded by the CHRM1 gene. It is localized to 11q13.

The muscarinic acetylcholine receptor M2, also known as the cholinergic receptor, muscarinic 2, is a muscarinic acetylcholine receptor that in humans is encoded by the CHRM2 gene. Multiple alternatively spliced transcript variants have been described for this gene. It is Gi-coupled, reducing intracellular levels of cAMP.

Oxaprotiline, also known as hydroxymaprotiline, is a norepinephrine reuptake inhibitor belonging to the tetracyclic antidepressant (TeCA) family and is related to maprotiline. Though investigated as an antidepressant, it was never marketed.

PD-102,807 is a drug which acts as a selective antagonist for the muscarinic acetylcholine receptor M4. It is used in scientific research for studying the effects of the different muscarinic receptor subtypes in the body and brain.
Autonomic drugs can either inhibit or enhance the functions of the parasympathetic and sympathetic nervous systems. This type of drug can be used to treat a wide range of diseases, such as glaucoma, asthma, urinary, gastrointestinal and cardiopulmonary disorders.

Cholinergic blocking drugs are a group of drugs that block the action of acetylcholine (ACh), a neurotransmitter, in synapses of the cholinergic nervous system. They block acetylcholine from binding to cholinergic receptors, namely the nicotinic and muscarinic receptors.


















