
Essential tremor (ET), also called benign tremor, familial tremor, and idiopathic tremor, is a medical condition characterized by involuntary rhythmic contractions and relaxations of certain muscle groups in one or more body parts of unknown cause. It is typically symmetrical, and affects the arms, hands, or fingers; but sometimes involves the head, vocal cords, or other body parts. Essential tremor is either an action (intention) tremor—it intensifies when one tries to use the affected muscles during voluntary movements such as eating and writing—or it is a postural tremor, present with sustained muscle tone. This means that it is distinct from a resting tremor, such as that caused by Parkinson's disease, which is not correlated with movement.

A tremor is an involuntary, somewhat rhythmic, muscle contraction and relaxation involving oscillations or twitching movements of one or more body parts. It is the most common of all involuntary movements and can affect the hands, arms, eyes, face, head, vocal folds, trunk, and legs. Most tremors occur in the hands. In some people, a tremor is a symptom of another neurological disorder.

MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) is an organic compound. It is classified as a tetrahydropyridine. It is of interest as a precursor to the neurotoxin MPP+, which causes permanent symptoms of Parkinson's disease by destroying dopaminergic neurons in the substantia nigra of the brain. It has been used to study disease models in various animals.
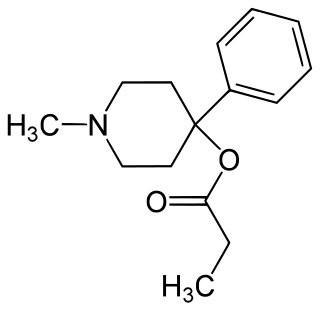
Desmethylprodine or 1-methyl-4-phenyl-4-propionoxypiperidine is an opioid analgesic drug developed in the 1940s by researchers at Hoffmann-La Roche. Desmethylprodine has been labeled by the DEA as a Schedule I drug in the United States. It is an analog of pethidine (meperidine) a Schedule II drug. Chemically, it is a reversed ester of pethidine which has about 70% of the potency of morphine. Unlike its derivative prodine, it was not reported to exhibit optical isomerism. It was reported to have 30 times the activity of pethidine and a greater analgesic effect than morphine in rats, and it was demonstrated to cause central nervous system stimulation in mice.
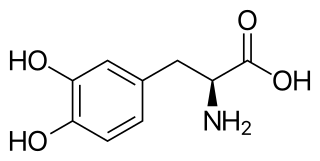
l-DOPA, also known as levodopa and l-3,4-dihydroxyphenylalanine, is made and used as part of the normal biology of some plants and animals, including humans. Humans, as well as a portion of the other animals that utilize l-DOPA, make it via biosynthesis from the amino acid l-tyrosine. l-DOPA is the precursor to the neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), which are collectively known as catecholamines. Furthermore, l-DOPA itself mediates neurotrophic factor release by the brain and CNS. In some plant families, l-DOPA is the central precursor of a biosynthetic pathway that produces a class of pigments called betalains. l-DOPA can be manufactured and in its pure form is sold as a psychoactive drug with the INN levodopa; trade names include Sinemet, Pharmacopa, Atamet, and Stalevo. As a drug, it is used in the clinical treatment of Parkinson's disease and dopamine-responsive dystonia.

Amantadine, sold under the brand name Gocovri among others, is a medication used to treat dyskinesia associated with parkinsonism and influenza caused by type A influenzavirus, though its use for the latter is no longer recommended because of widespread drug resistance. It acts as a nicotinic antagonist, dopamine agonist, and noncompetitive NMDA antagonist. The antiviral mechanism of action is antagonism of the influenzavirus A M2 proton channel, which prevents endosomal escape.

Glycopyrronium bromide is a medication of the muscarinic anticholinergic group. It does not cross the blood–brain barrier and consequently has few to no central effects. It is given by mouth, via intravenous injection, on the skin, and via inhalation. It is a synthetic quaternary ammonium compound. The cation, which is the active moiety, is called glycopyrronium (INN) or glycopyrrolate (USAN).
Hypokinesia is one of the classifications of movement disorders, and refers to decreased bodily movement. Hypokinesia is characterized by a partial or complete loss of muscle movement due to a disruption in the basal ganglia. Hypokinesia is a symptom of Parkinson's disease shown as muscle rigidity and an inability to produce movement. It is also associated with mental health disorders and prolonged inactivity due to illness, amongst other diseases.

Chlorprothixene, sold under the brand name Truxal among others, is a typical antipsychotic of the thioxanthene group.

Biperiden, sold under the brand name Akineton among others, is a medication used to treat Parkinson disease, certain drug-induced movement disorders and Tourette Syndrome. It is not recommended for tardive dyskinesias. It is taken by mouth, injection into a vein, or muscle.

Trihexyphenidyl is an antispasmodic drug used to treat stiffness, tremors, spasms, and poor muscle control. It is an agent of the antimuscarinic class and is often used in management of Parkinson's disease. It was approved by the FDA for the treatment of Parkinson's in the US in 2003.

Oxybutynin, sold as under the brand name Ditropan among others, is an anticholinergic drug primarily used to treat overactive bladder. It is widely considered a first-line therapy for overactive bladder due to its well-studied side effect profile, broad applicability, and continued efficacy over long periods of time. It works similar to tolterodine, darifenacin, and solifenacin, although it is usually preferred over these medications. It is sometimes used off-label for treatment of hyperhidrosis, or excessive sweating. It has also been used off-label to treat bed wetting in children, but this use has declined, as it is most likely ineffective in this role. It is taken by mouth or applied to the skin.
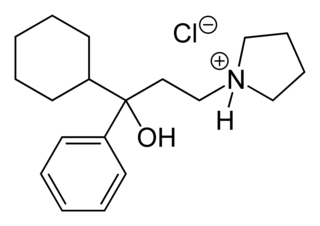
Procyclidine is an anticholinergic drug principally used for the treatment of drug-induced parkinsonism, akathisia and acute dystonia, Parkinson's disease, and idiopathic or secondary dystonia.
In the management of Parkinson's disease, due to the chronic nature of Parkinson's disease (PD), a broad-based program is needed that includes patient and family education, support-group services, general wellness maintenance, exercise, and nutrition. At present, no cure for the disease is known, but medications or surgery can provide relief from the symptoms.

Budipine is an antiparkinson agent marketed for the treatment of Parkinson's disease.
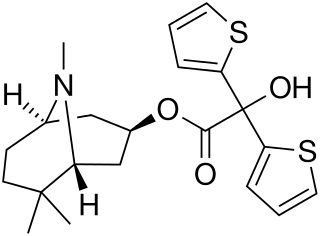
Mazaticol (Pentona) is an anticholinergic used as an antiparkinsonian agent in Japan.
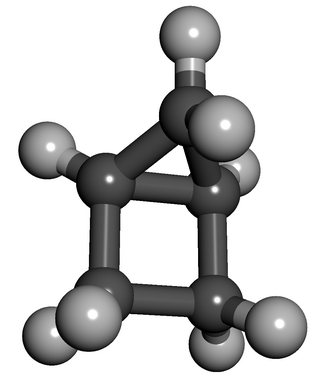
Housane or bicyclo[2.1.0]pentane is a saturated cycloalkane with the formula C5H8. It is a colorless, volatile liquid at room temperature. It was named "housane" because of its shape, which resembles a simple drawing of a house. Structurally, the molecule consists of cyclopropane fused to cyclobutane. The synthesis of molecules containing multiple strained rings, such as housane, is a traditional endeavor in synthetic organic chemistry.
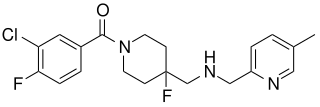
Befiradol is an experimental drug being studied for the treatment of levodopa-induced dyskinesia. It is a potent and selective 5-HT1A receptor full agonist.
EXP-561 is an investigational drug that acts as an inhibitor of the reuptake of serotonin, dopamine, and norepinephrine. It was developed in the 1960s by Du Pont and was suggested as a potential antidepressant but failed in trials and was never marketed.

Sofpironium bromide is a drug used to treat hyperhidrosis. It was approved in Japan in 2020 as a 5% gel for the treatment of primary axillary hyperhidrosis (PAH).




















