
Histone acetyltransferases (HATs) are enzymes that acetylate conserved lysine amino acids on histone proteins by transferring an acetyl group from acetyl-CoA to form ε-N-acetyllysine. DNA is wrapped around histones, and, by transferring an acetyl group to the histones, genes can be turned on and off. In general, histone acetylation increases gene expression.
Drug metabolism is the metabolic breakdown of drugs by living organisms, usually through specialized enzymatic systems. More generally, xenobiotic metabolism is the set of metabolic pathways that modify the chemical structure of xenobiotics, which are compounds foreign to an organism's normal biochemistry, such as any drug or poison. These pathways are a form of biotransformation present in all major groups of organisms and are considered to be of ancient origin. These reactions often act to detoxify poisonous compounds. The study of drug metabolism is called pharmacokinetics.

Histone deacetylases (EC 3.5.1.98, HDAC) are a class of enzymes that remove acetyl groups (O=C-CH3) from an ε-N-acetyl lysine amino acid on both histone and non-histone proteins. HDACs allow histones to wrap the DNA more tightly. This is important because DNA is wrapped around histones, and DNA expression is regulated by acetylation and de-acetylation. HDAC's action is opposite to that of histone acetyltransferase. HDAC proteins are now also called lysine deacetylases (KDAC), to describe their function rather than their target, which also includes non-histone proteins. In general, they suppress gene expression.

Chloramphenicol acetyltransferase is a bacterial enzyme that detoxifies the antibiotic chloramphenicol and is responsible for chloramphenicol resistance in bacteria. This enzyme covalently attaches an acetyl group from acetyl-CoA to chloramphenicol, which prevents chloramphenicol from binding to ribosomes. A histidine residue, located in the C-terminal section of the enzyme, plays a central role in its catalytic mechanism.

Iproniazid is a non-selective, irreversible monoamine oxidase inhibitor (MAOI) of the hydrazine class. It is a xenobiotic that was originally designed to treat tuberculosis, but was later most prominently used as an antidepressant drug. However, it was withdrawn from the market because of its hepatotoxicity. The medical use of iproniazid was discontinued in most of the world in the 1960s, but remained in use in France until its discontinuation in 2015.
4-Aminobiphenyl (4-ABP) is an organic compound with the formula C6H5C6H4NH2. It is an amine derivative of biphenyl. It is a colorless solid, although aged samples can appear colored. 4-Aminobiphenyl was commonly used in the past as a rubber antioxidant and an intermediate for dyes. Exposure to this aryl-amine can happen through contact with chemical dyes and from inhalation of cigarette smoke. Researches showed that 4-aminobiphenyl is responsible for bladder cancer in humans and dogs by damaging DNA. Due to its carcinogenic effects, commercial production of 4-aminobiphenyl ceased in the United States in the 1950s.

Acecainide is an antiarrhythmic drug. Chemically, it is the N-acetylated metabolite of procainamide. It is a Class III antiarrhythmic agent, whereas procainamide is a Class Ia antiarrhythmic drug. It is only partially as active as procainamide; when checking levels, both must be included in the final calculation.

Acetyltransferase is a type of transferase enzyme that transfers an acetyl group, through a process called acetylation. Acetylation serves as a modification that can profoundly transform the functionality of a protein by modifying various properties like hydrophobicity, solubility, and surface attributes. These alterations have the potential to influence the protein's conformation and its interactions with substrates, cofactors, and other macromolecules. The image to the right shows the basic structure of an acetyl group, where R is a variable indicates the remainder of the molecule to which the acetyl group is attached.

Histone acetylation and deacetylation are the processes by which the lysine residues within the N-terminal tail protruding from the histone core of the nucleosome are acetylated and deacetylated as part of gene regulation.

N-acetyltransferase 2 , also known as NAT2, is an enzyme which in humans is encoded by the NAT2 gene.
In enzymology, an alpha-tubulin N-acetyltransferase is an enzyme which is encoded by the ATAT1 gene.
In enzymology, an arylamine N-acetyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, glucosamine-phosphate N-acetyltransferase (GNA) is an enzyme that catalyzes the transfer of an acetyl group from acetyl-CoA to the primary amine in glucosamide-6-phosphate, generating a free CoA and N-acetyl-D-glucosamine-6-phosphate.
In enzymology, a peptide alpha-N-acetyltransferase is an enzyme that catalyzes the chemical reaction

N-alpha-acetyltransferase 10 (NAA10) also known as NatA catalytic subunit Naa10 and arrest-defective protein 1 homolog A (ARD1A) is an enzyme subunit that in humans is encoded NAA10 gene. Together with its auxiliary subunit Naa15, Naa10 constitutes the NatA complex that specifically catalyzes the transfer of an acetyl group from acetyl-CoA to the N-terminal primary amino group of certain proteins. In higher eukaryotes, 5 other N-acetyltransferase (NAT) complexes, NatB-NatF, have been described that differ both in substrate specificity and subunit composition.
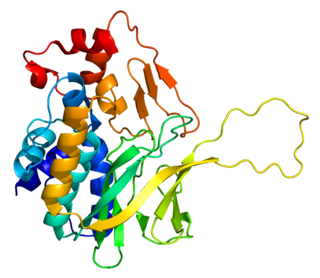
N-acetyltransferase 1 is a protein that in humans is encoded by the NAT1 gene.
Protein acetylation are acetylation reactions that occur within living cells as drug metabolism, by enzymes in the liver and other organs. Pharmaceuticals frequently employ acetylation to enable such esters to cross the blood–brain barrier, where they are deacetylated by enzymes (carboxylesterases) in a manner similar to acetylcholine. Examples of acetylated pharmaceuticals are diacetylmorphine (heroin), acetylsalicylic acid (aspirin), THC-O-acetate, and diacerein. Conversely, drugs such as isoniazid are acetylated within the liver during drug metabolism. A drug that depends on such metabolic transformations in order to act is termed a prodrug.

2-Aminofluorene (2-AF) is a synthetic arylamine. It is a white to tan solid with a melting point of 125-132 °C. 2-AF has only been tested in controlled laboratory settings thus far. There is no indication that it will be tested in industrialized settings. There is evidence that 2-aminofluorene is a carcinogen and an intercalating agent that is extremely dangerous to genomic DNA that potentially can lead to mutation if not death. Furthermore, it has been suggested that 2-aminofluorene can undergo acetylation reactions that causes these reactive species to undergo such reactions in cells. Several experiments have been conducted that have suggested 2-aminofluorene be treated with care and with an overall awareness of the toxicity of this compound.

Urs Albert Meyer is a Swiss physician-scientist and clinical pharmacologist.

Nourseothricin (NTC) is a member of the streptothricin-class of aminoglycoside antibiotics produced by Streptomyces species. Chemically, NTC is a mixture of the related compounds streptothricin C, D, E, and F. NTC inhibits protein synthesis by inducing miscoding. It is used as a selection marker for a wide range of organisms including bacteria, yeast, filamentous fungi, and plant cells. It is not known to have adverse side-effects on positively selected cells, a property cardinal to a selection drug.
















