Ubiquitin is a small regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ubiquitously. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Four genes in the human genome code for ubiquitin: UBB, UBC, UBA52 and RPS27A.
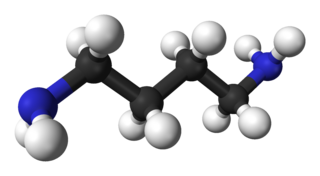
Putrescine is an organic compound with the formula (CH2)4(NH2)2. It is a colorless solid that melts near room temperature. It is classified as a diamine. Together with cadaverine, it is largely responsible for the foul odor of putrefying flesh, but also contributes to other unpleasant odors.

A ubiquitin ligase is a protein that recruits an E2 ubiquitin-conjugating enzyme that has been loaded with ubiquitin, recognizes a protein substrate, and assists or directly catalyzes the transfer of ubiquitin from the E2 to the protein substrate. In simple and more general terms, the ligase enables movement of ubiquitin from a ubiquitin carrier to another thing by some mechanism. The ubiquitin, once it reaches its destination, ends up being attached by an isopeptide bond to a lysine residue, which is part of the target protein. E3 ligases interact with both the target protein and the E2 enzyme, and so impart substrate specificity to the E2. Commonly, E3s polyubiquitinate their substrate with Lys48-linked chains of ubiquitin, targeting the substrate for destruction by the proteasome. However, many other types of linkages are possible and alter a protein's activity, interactions, or localization. Ubiquitination by E3 ligases regulates diverse areas such as cell trafficking, DNA repair, and signaling and is of profound importance in cell biology. E3 ligases are also key players in cell cycle control, mediating the degradation of cyclins, as well as cyclin dependent kinase inhibitor proteins. The human genome encodes over 600 putative E3 ligases, allowing for tremendous diversity in substrates.

Eflornithine, sold under the brand name Vaniqa among others, is a medication used to treat African trypanosomiasis and excessive hair growth on the face in women. Specifically it is used for the 2nd stage of sleeping sickness caused by T. b. gambiense and may be used with nifurtimox. It is taken intravenously or topically. It has also been given orally on at least some rare occasions for the treatment of African trypanosomiasis.
Spermine is a polyamine involved in cellular metabolism that is found in all eukaryotic cells. The precursor for synthesis of spermine is the amino acid ornithine. It is an essential growth factor in some bacteria as well. It is found as a polycation at physiological pH. Spermine is associated with nucleic acids and is thought to stabilize helical structure, particularly in viruses. It functions as an intracellular free radical scavenger to protect DNA from free radical attack. Spermine is the chemical primarily responsible for the characteristic odor of semen.

Spermidine is a polyamine compound found in ribosomes and living tissues and having various metabolic functions within organisms. It was originally isolated from semen.

Spermidine synthase is an enzyme that catalyzes the transfer of the propylamine group from S-adenosylmethioninamine to putrescine in the biosynthesis of spermidine. The systematic name is S-adenosyl 3-(methylthio)propylamine:putrescine 3-aminopropyltransferase and it belongs to the group of aminopropyl transferases. It does not need any cofactors. Most spermidine synthases exist in solution as dimers.

A degron is a portion of a protein that is important in regulation of protein degradation rates. Known degrons include short amino acid sequences, structural motifs and exposed amino acids located anywhere in the protein. In fact, some proteins can even contain multiple degrons. Degrons are present in a variety of organisms, from the N-degrons first characterized in yeast to the PEST sequence of mouse ornithine decarboxylase. Degrons have been identified in prokaryotes as well as eukaryotes. While there are many types of different degrons, and a high degree of variability even within these groups, degrons are all similar for their involvement in regulating the rate of a protein's degradation. Much like protein degradation, mechanisms are categorized by their dependence or lack thereof on ubiquitin, a small protein involved in proteasomal protein degradation, Degrons may also be referred to as “ubiquitin-dependent" or “ubiquitin-independent".
Ubiquitin-conjugating enzymes, also known as E2 enzymes and more rarely as ubiquitin-carrier enzymes, perform the second step in the ubiquitination reaction that targets a protein for degradation via the proteasome. The ubiquitination process covalently attaches ubiquitin, a short protein of 76 amino acids, to a lysine residue on the target protein. Once a protein has been tagged with one ubiquitin molecule, additional rounds of ubiquitination form a polyubiquitin chain that is recognized by the proteasome's 19S regulatory particle, triggering the ATP-dependent unfolding of the target protein that allows passage into the proteasome's 20S core particle, where proteases degrade the target into short peptide fragments for recycling by the cell.
SpeF is a putative cis-acting element identified in several gram negative alpha proteobacteria. It is proposed to be involved in regulating expression of genes involved in polyamide biosynthesis.

In molecular biology, Ornithine decarboxylase antizyme (ODC-AZ) is an ornithine decarboxylase inhibitor. It binds to, and destabilises, ornithine decarboxylase (ODC), a key enzyme in polyamine synthesis. ODC is then rapidly degraded. It was first characterized in 1981. The expression of ODC-AZ requires programmed, ribosomal frameshifting which is modulated according to the cellular concentration of polyamines. High levels of polyamines induce a +1 ribosomal frameshift in the translation of mRNA for the antizyme leading to the expression of a full-length protein. At least two forms of ODC-AZ exist in mammals and the protein has been found in Drosophila as well as in Saccharomyces yeast.

26S proteasome non-ATPase regulatory subunit 13 is an enzyme that in humans is encoded by the PSMD13 gene.

26S proteasome non-ATPase regulatory subunit 11 is an enzyme that in humans is encoded by the PSMD11 gene.

Diamine acetyltransferase 1 is an enzyme that in humans is encoded by the SAT1 gene found on the X chromosome.

Ornithine decarboxylase antizyme is an enzyme that in humans is encoded by the OAZ1 gene.

26S proteasome non-ATPase regulatory subunit 14, also known as 26S proteasome non-ATPase subunit Rpn11, is an enzyme that in humans is encoded by the PSMD14 gene. This protein is one of the 19 essential subunits of the complete assembled 19S proteasome complex. Nine subunits Rpn3, Rpn5, Rpn6, Rpn7, Rpn8, Rpn9, Rpn11, SEM1, and Rpn12 form the lid sub complex of the 19S regulatory particle of the proteasome complex.

Antizyme inhibitor 1 is a protein that in humans is encoded by the AZIN1 gene.

Ornithine decarboxylase antizyme 2 is an enzyme that in humans is encoded by the OAZ2 gene.
A polyamine is an organic compound having more than two amino groups. Alkyl polyamines occur naturally, but some are synthetic. Alkylpolyamines are colorless, hygroscopic, and water soluble. Near neutral pH, they exist as the ammonium derivatives. Most aromatic polyamines are crystalline solids at room temperature.
Bachmann-Bupp Syndrome (BABS) is a rare genetic disorder linked to mutations in the ODC1 gene. It is caused by 3' end mutations of the ODC1 gene which produces C-terminally truncated variants of ODC, a pyridoxal 5'-phosphate-dependent enzyme. This prevents ubiquitin-independent proteasomal degradation and leads to cellular accumulation of ODC. This leads to an increased conversion of ornithine to putrescine, causing an accumulation of putrescine. So, if the ODC protein is not properly degraded leading to an accumulation ODC and later putrescine, it causes many gain-of-function mutations. This leads to a wide variety of symptoms which is why misdiagnosis is often a reality. Penetrance of the pathogenic variants for the ODC1 gene is believed to be 100%.