Glycolysis is the metabolic pathway that converts glucose into pyruvate and, in most organisms, occurs in the liquid part of cells. The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes.
Gluconeogenesis (GNG) is a metabolic pathway that results in the biosynthesis of glucose from certain non-carbohydrate carbon substrates. It is a ubiquitous process, present in plants, animals, fungi, bacteria, and other microorganisms. In vertebrates, gluconeogenesis occurs mainly in the liver and, to a lesser extent, in the cortex of the kidneys. It is one of two primary mechanisms – the other being degradation of glycogen (glycogenolysis) – used by humans and many other animals to maintain blood sugar levels, avoiding low levels (hypoglycemia). In ruminants, because dietary carbohydrates tend to be metabolized by rumen organisms, gluconeogenesis occurs regardless of fasting, low-carbohydrate diets, exercise, etc. In many other animals, the process occurs during periods of fasting, starvation, low-carbohydrate diets, or intense exercise.

The enzyme fructose bisphosphatase (EC 3.1.3.11; systematic name D-fructose-1,6-bisphosphate 1-phosphohydrolase) catalyses the conversion of fructose-1,6-bisphosphate to fructose 6-phosphate in gluconeogenesis and the Calvin cycle, which are both anabolic pathways:

Tumor hypoxia is the situation where tumor cells have been deprived of oxygen. As a tumor grows, it rapidly outgrows its blood supply, leaving portions of the tumor with regions where the oxygen concentration is significantly lower than in healthy tissues. Hypoxic microenvironments in solid tumors are a result of available oxygen being consumed within 70 to 150 μm of tumor vasculature by rapidly proliferating tumor cells thus limiting the amount of oxygen available to diffuse further into the tumor tissue. In order to support continuous growth and proliferation in challenging hypoxic environments, cancer cells are found to alter their metabolism. Furthermore, hypoxia is known to change cell behavior and is associated with extracellular matrix remodeling and increased migratory and metastatic behavior.

Phosphofructokinase-1 (PFK-1) is one of the most important regulatory enzymes of glycolysis. It is an allosteric enzyme made of 4 subunits and controlled by many activators and inhibitors. PFK-1 catalyzes the important "committed" step of glycolysis, the conversion of fructose 6-phosphate and ATP to fructose 1,6-bisphosphate and ADP. Glycolysis is the foundation for respiration, both anaerobic and aerobic. Because phosphofructokinase (PFK) catalyzes the ATP-dependent phosphorylation to convert fructose-6-phosphate into fructose 1,6-bisphosphate and ADP, it is one of the key regulatory steps of glycolysis. PFK is able to regulate glycolysis through allosteric inhibition, and in this way, the cell can increase or decrease the rate of glycolysis in response to the cell's energy requirements. For example, a high ratio of ATP to ADP will inhibit PFK and glycolysis. The key difference between the regulation of PFK in eukaryotes and prokaryotes is that in eukaryotes PFK is activated by fructose 2,6-bisphosphate. The purpose of fructose 2,6-bisphosphate is to supersede ATP inhibition, thus allowing eukaryotes to have greater sensitivity to regulation by hormones like glucagon and insulin.

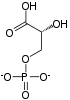
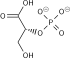
Pyruvate kinase is the enzyme involved in the last step of glycolysis. It catalyzes the transfer of a phosphate group from phosphoenolpyruvate (PEP) to adenosine diphosphate (ADP), yielding one molecule of pyruvate and one molecule of ATP. Pyruvate kinase was inappropriately named before it was recognized that it did not directly catalyze phosphorylation of pyruvate, which does not occur under physiological conditions. Pyruvate kinase is present in four distinct, tissue-specific isozymes in animals, each consisting of particular kinetic properties necessary to accommodate the variations in metabolic requirements of diverse tissues.

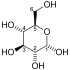
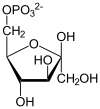
Glucose 6-phosphate is a glucose sugar phosphorylated at the hydroxy group on carbon 6. This dianion is very common in cells as the majority of glucose entering a cell will become phosphorylated in this way.
Glucose-6-phosphate isomerase (GPI), alternatively known as phosphoglucose isomerase/phosphoglucoisomerase (PGI) or phosphohexose isomerase (PHI), is an enzyme that in humans is encoded by the GPI gene on chromosome 19. This gene encodes a member of the glucose phosphate isomerase protein family. The encoded protein has been identified as a moonlighting protein based on its ability to perform mechanistically distinct functions. In the cytoplasm, the gene product functions as a glycolytic enzyme that interconverts glucose-6-phosphate (G6P) and fructose-6-phosphate (F6P). Extracellularly, the encoded protein functions as a neurotrophic factor that promotes survival of skeletal motor neurons and sensory neurons, and as a lymphokine that induces immunoglobulin secretion. The encoded protein is also referred to as autocrine motility factor (AMF) based on an additional function as a tumor-secreted cytokine and angiogenic factor. Defects in this gene are the cause of nonspherocytic hemolytic anemia, and a severe enzyme deficiency can be associated with hydrops fetalis, immediate neonatal death and neurological impairment. Alternative splicing results in multiple transcript variants. [provided by RefSeq, Jan 2014]

Phosphofructokinase-2 (6-phosphofructo-2-kinase, PFK-2) or fructose bisphosphatase-2 (FBPase-2), is an enzyme indirectly responsible for regulating the rates of glycolysis and gluconeogenesis in cells. It catalyzes formation and degradation of a significant allosteric regulator, fructose-2,6-bisphosphate (Fru-2,6-P2) from substrate fructose-6-phosphate. Fru-2,6-P2 contributes to the rate-determining step of glycolysis as it activates enzyme phosphofructokinase 1 in the glycolysis pathway, and inhibits fructose-1,6-bisphosphatase 1 in gluconeogenesis. Since Fru-2,6-P2 differentially regulates glycolysis and gluconeogenesis, it can act as a key signal to switch between the opposing pathways. Because PFK-2 produces Fru-2,6-P2 in response to hormonal signaling, metabolism can be more sensitively and efficiently controlled to align with the organism's glycolytic needs. This enzyme participates in fructose and mannose metabolism. The enzyme is important in the regulation of hepatic carbohydrate metabolism and is found in greatest quantities in the liver, kidney and heart. In mammals, several genes often encode different isoforms, each of which differs in its tissue distribution and enzymatic activity. The family described here bears a resemblance to the ATP-driven phospho-fructokinases; however, they share little sequence similarity, although a few residues seem key to their interaction with fructose 6-phosphate.

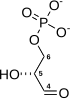
Fructose 1,6-bisphosphate, known in older publications as Harden-Young ester, is fructose sugar phosphorylated on carbons 1 and 6. The β-D-form of this compound is common in cells. Upon entering the cell, most glucose and fructose is converted to fructose 1,6-bisphosphate.

Aldolase B also known as fructose-bisphosphate aldolase B or liver-type aldolase is one of three isoenzymes of the class I fructose 1,6-bisphosphate aldolase enzyme, and plays a key role in both glycolysis and gluconeogenesis. The generic fructose 1,6-bisphosphate aldolase enzyme catalyzes the reversible cleavage of fructose 1,6-bisphosphate (FBP) into glyceraldehyde 3-phosphate and dihydroxyacetone phosphate (DHAP) as well as the reversible cleavage of fructose 1-phosphate (F1P) into glyceraldehyde and dihydroxyacetone phosphate. In mammals, aldolase B is preferentially expressed in the liver, while aldolase A is expressed in muscle and erythrocytes and aldolase C is expressed in the brain. Slight differences in isozyme structure result in different activities for the two substrate molecules: FBP and fructose 1-phosphate. Aldolase B exhibits no preference and thus catalyzes both reactions, while aldolases A and C prefer FBP.

Aldolase A deficiency is an autosomal recessive metabolic disorder resulting in a deficiency of the enzyme aldolase A; the enzyme is found predominantly in red blood cells and muscle tissue. The deficiency may lead to hemolytic anaemia as well as myopathy associated with exercise intolerance and rhabdomyolysis in some cases.

Fructose-bisphosphate aldolase, often just aldolase, is an enzyme catalyzing a reversible reaction that splits the aldol, fructose 1,6-bisphosphate, into the triose phosphates dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (G3P). Aldolase can also produce DHAP from other (3S,4R)-ketose 1-phosphates such as fructose 1-phosphate and sedoheptulose 1,7-bisphosphate. Gluconeogenesis and the Calvin cycle, which are anabolic pathways, use the reverse reaction. Glycolysis, a catabolic pathway, uses the forward reaction. Aldolase is divided into two classes by mechanism.
Glucose-1,6-bisphosphate synthase is a type of enzyme called a phosphotransferase and is involved in mammalian starch and sucrose metabolism. It catalyzes the transfer of a phosphate group from 1,3-bisphosphoglycerate to glucose-1-phosphate, yielding 3-phosphoglycerate and glucose-1,6-bisphosphate.

6-phosphofructokinase, liver type (PFKL) is an enzyme that in humans is encoded by the PFKL gene on chromosome 21. This gene encodes the liver (L) isoform of phosphofructokinase-1, an enzyme that catalyzes the conversion of D-fructose 6-phosphate to D-fructose 1,6-bisphosphate, which is a key step in glucose metabolism (glycolysis). This enzyme is a tetramer that may be composed of different subunits encoded by distinct genes in different tissues. Alternative splicing results in multiple transcript variants. [provided by RefSeq, Mar 2014]

Phosphofructokinase, platelet, also known as PFKP is an enzyme which in humans is encoded by the PFKP gene.

6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 2 is an enzyme that in humans is encoded by the PFKFB2 gene.

Aldolase C, fructose-bisphosphate, is an enzyme that, in humans, is encoded by the ALDOC gene on chromosome 17. This gene encodes a member of the class I fructose-bisphosphate aldolase gene family. Expressed specifically in the hippocampus and Purkinje cells of the brain, the encoded protein is a glycolytic enzyme that catalyzes the reversible aldol cleavage of fructose 1,6-bisphosphate and fructose-1-phosphate to dihydroxyacetone phosphate and either glyceraldehyde 3-phosphate or glyceraldehyde, respectively.[provided by RefSeq, Jul 2008]
Pyruvate kinase PKLR is an enzyme that in humans is encoded by the PKLR gene.

The TP53-inducible glycolysis and apoptosis regulator (TIGAR) also known as fructose-2,6-bisphosphatase TIGAR is an enzyme that in humans is encoded by the C12orf5 gene.